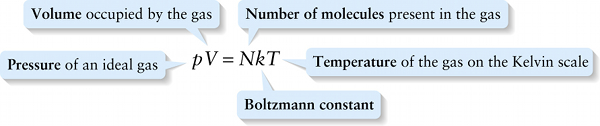
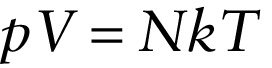Ideal gas law in terms of number of molecules (14-6)
Question 1 of 5
Question
Volume occupied by the gas
{"title":"Volume occupied by the gas","description":"Correct!","type":"correct","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"rect\",\"coords\":\"37,5,82,51\"}]"} {"title":"Number of molecules present in the gas","description":"Wrong","type":"incorrect","color":"#ffcc00","code":"[{\"shape\":\"rect\",\"coords\":\"137,7,187,52\"}]"} {"title":"Temperature of the gas on the Kelvin scale","description":"Incorrect","type":"incorrect","color":"#333300","code":"[{\"shape\":\"rect\",\"coords\":\"218,2,262,51\"}]"} {"title":"Boltzmann constant","description":"Incorrect","type":"incorrect","color":"#000080","code":"[{\"shape\":\"rect\",\"coords\":\"189,4,218,53\"}]"} {"title":"Pressure of an ideal gas","description":"Wrong","type":"incorrect","color":"#333333","code":"[{\"shape\":\"rect\",\"coords\":\"1,13,31,65\"}]"}Review
The constant k in Equation 14-6, called the Boltzmann constant, turns out to have the same value for all gases. To four significant figures,
k=1.381×10−23J/K
Equation 14-6 is called the ideal gas law, and a gas that would obey this equation exactly is called an ideal gas. Real gases are not ideal: They do not obey this equation exactly, especially at high pressures or at temperatures close to the point at which the gases become liquids. At relatively low gas densities, however, the ideal gas law does a good job of representing the relationship between pressure, volume, and temperature for real gases. That’s why an ideal gas is a useful idealization.