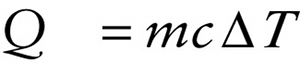Quantity of heat and the resulting temperature change (14-20)
Question
Quantity of heat that flows into (if Q > 0) or out of (Q < 0) an object
{"title":"Quantity of heat that flows into (if Q > 0) or out of (Q < 0) an object","description":"Correct!","type":"correct","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"rect\",\"coords\":\"2,6,54,61\"}]"} {"title":"Specific heat of the substance of which the object is made","description":"Wrong","type":"incorrect","color":"#ffcc00","code":"[{\"shape\":\"rect\",\"coords\":\"191,19,217,52\"}]"} {"title":"Temperature change of the object that results from the heat flow","description":"Incorrect","type":"incorrect","color":"#333300","code":"[{\"shape\":\"rect\",\"coords\":\"264,8,299,51\"}]"} {"title":"Mass of the object","description":"Incorrect","type":"incorrect","color":"#000080","code":"[{\"shape\":\"rect\",\"coords\":\"145,17,188,51\"}]"}Review
Experiment shows that in most circumstances the flow of heat into or out of an object changes the object’s temperature. (The exception is when the object undergoes a phase change such as from solid to liquid, liquid to solid, liquid to gas, or gas to liquid. In the next section we’ll discuss what happens in a phase change.) If the quantity of heat Q that flows into an object is rela tively small, the resulting temperature change ΔT turns out to be directly proportional to Q and inversely proportional to the mass m of the object. In other words, the greater the quantity of heat Q that flows into an object, the more its temperature changes; the more massive the object and so the more material that makes up the object, the smaller the temperature change for a given quantity of heat Q. (The same quantity of heat that will cook a single meatball will cause hardly any temperature change in a pot roast.) We can write this relationship as
ΔT=(constant)×Qm
The constant in this equation depends on the substance of which the object is made. It’s conventional to express this equation as
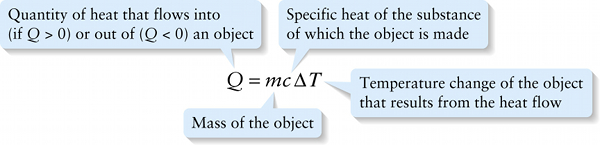
The quantity c is called the specific heat of the material that makes up the object. Its units are joules per kilogram per Kelvin (J/(kg⋅K) or J⋅kg−1⋅K−1) For example, the value of c for aluminum is 910 J/(kg⋅K); this means that 910 J of heat must flow into a 1-kg block of aluminum to raise its temperature by 1 K (or, equivalently, 1∘C).