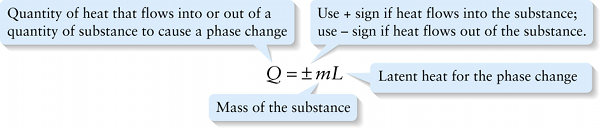
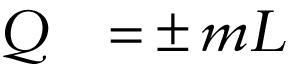Heat required for a phase change (14-21)
Question 1 of 4
Question
Mass of the substance
{"title":"Quantity of heat that flows into or out of a quantity of substance to cause a phase change","description":"Wrong","type":"incorrect","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"rect\",\"coords\":\"2,10,54,65\"}]"} {"title":"Use + sign if heat flows into the substance; use – sign if heat flows out of the substance.","description":"Wrong","type":"incorrect","color":"#ffcc00","code":"[{\"shape\":\"rect\",\"coords\":\"154,12,192,53\"}]"} {"title":"Latent heat for the phase change","description":"Incorrect","type":"incorrect","color":"#333300","code":"[{\"shape\":\"rect\",\"coords\":\"251,8,286,51\"}]"} {"title":"Mass of the substance","description":"Correct!","type":"correct","color":"#000080","code":"[{\"shape\":\"rect\",\"coords\":\"202,19,245,53\"}]"}Review
The amount of energy needed to cause a phase change to occur is proportional to the mass m of substance. The more massive a block of ice or iron, for example, the more energy is required to melt it. We can summarize these relationships in a single equation: