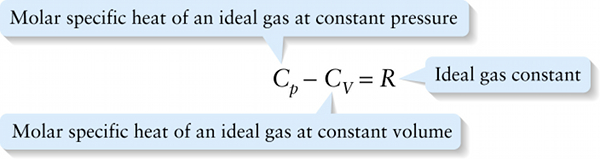
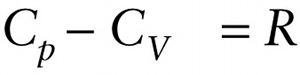Molar specific heats of an ideal gas (15-15)
Question 1 of 3
Question
Molar specific heat of an ideal gas at constant volume
{"title":"Molar specific heat of an ideal gas at constant pressure","description":"Incorrect","type":"incorrect","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"rect\",\"coords\":\"1,7,42,51\"},{\"shape\":\"poly\",\"coords\":\"144,22\"}]"} {"title":"Ideal gas constant","description":"Incorrect","type":"incorrect","color":"#ffcc00","code":"[{\"shape\":\"rect\",\"coords\":\"259,6,297,52\"}]"} {"title":"Molar specific heat of an ideal gas at constant volume","description":"Correct!","type":"correct","color":"#333300","code":"[{\"shape\":\"rect\",\"coords\":\"110,6,151,47\"}]"}Review
Equation 15-15 says that for an ideal gas, Cp is greater than CV by R=8.314J/(mol⋅K), the ideal gas constant. The experimentally determined values of Cp−CV given in Table 15-1 for real gases are very close to this theoretical prediction.