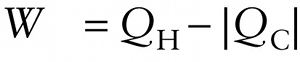Work done by a heat engine (15-24)
Question 1 of 3
Question
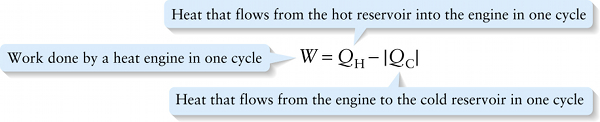
Heat that flows from the hot reservoir into the engine in one cycle
{"title":"Work done by a heat engine in one cycle","description":"Wrong","type":"incorrect","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"poly\",\"coords\":\"144,22\"},{\"shape\":\"rect\",\"coords\":\"5,4,45,38\"}]"} {"title":"Heat that flows from the hot reservoir into the engine in one cycle","description":"Correct!","type":"correct","color":"#ffcc00","code":"[{\"shape\":\"rect\",\"coords\":\"113,4,156,49\"}]"} {"title":"Heat that flows from the engine to the cold reservoir in one cycle","description":"Wrong","type":"incorrect","color":"#333300","code":"[{\"shape\":\"rect\",\"coords\":\"232,2,273,54\"}]"}Review
Let’s apply the first law of thermodynamics, Equation 15-2, to the generic engine shown in Figure 15-14. In one cycle of the engine it takes in an amount of energy QH from the hot reservoir in the form of heat and sends an amount of energy |QC|to the cold reservoir in the form of heat, so the net quantity of heat that goes into the engine is Q=QH−|QC|. In that same cycle the engine does an amount of work W. Because the engine goes through a cycle that returns it to its initial state at the beginning of the cycle, there is zero net change in its internal energy: ΔU=0. So the first law of thermodynamics, ΔU=Q−W, becomes
0=QH−|QC|−W
or