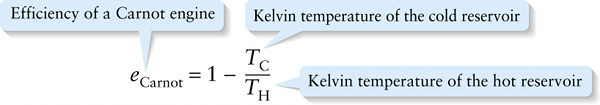
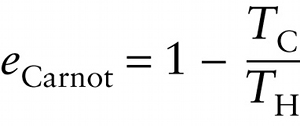Efficiency of a Carnot engine (15-28)
Question 1 of 3
Question
Efficiency of a Carnot engine
{"title":"Efficiency of a Carnot engine","description":"Correct!","type":"correct","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"poly\",\"coords\":\"144,22\"},{\"shape\":\"rect\",\"coords\":\"23,63,116,92\"}]"} {"title":"Kelvin temperature of the cold reservoir","description":"Wrong","type":"incorrect","color":"#ffcc00","code":"[{\"shape\":\"rect\",\"coords\":\"243,2,278,41\"}]"} {"title":"Kelvin temperature of the hot reservoir ","description":"Wrong","type":"incorrect","color":"#333300","code":"[{\"shape\":\"rect\",\"coords\":\"244,63,279,107\"}]"}Review
The efficiency of an ideal Carnot heat engine depends only on the hot and cold temperatures between which the engine operates. Equation 15-28 is the maximum theoretical efficiency of any heat engine operating between temperatures TH and TC. It is impossible for any engine to do better than this.