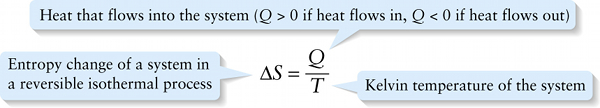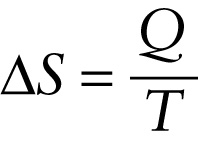Entropy change in a reversible isothermal process (15-33)
Question 1 of 3
Question
Heat that flows into the system (Q>0 if heat flows in, Q<0 if heat flows out)
{"title":"Entropy change of a system in a reversible isothermal process","description":"Incorrect","type":"incorrect","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"poly\",\"coords\":\"144,22\"},{\"shape\":\"rect\",\"coords\":\"33,50,69,99\"}]"} {"title":"Heat that flows into the system (Q > 0 if heat flows in, Q < 0 if heat flows out)","description":"Correct!","type":"correct","color":"#ffcc00","code":"[{\"shape\":\"rect\",\"coords\":\"137,4,194,64\"}]"} {"title":"Kelvin temperature of the system","description":"Wrong","type":"incorrect","color":"#333300","code":"[{\"shape\":\"rect\",\"coords\":\"142,86,188,133\"}]"}Review
The entropy change ΔS in an isothermal expansion also depends on the temperature T. For a given quantity of heat Q, the increase in disorder is greater if the system is at a low temperature (so the molecules are moving slowly and are relatively well ordered to start with) than if the system is at a high temperature (so the molecules are moving rapidly in a relatively disordered state). We conclude that the entropy change ΔS in this reversible isothermal process is inversely proportional to the Kelvin temperature T.
Putting these ideas together, we define the entropy change ΔS in a reversible isothermal process as