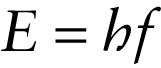Energy of a photon (22-26)
Question
Planck’s constant = 6.62606957 \times 10^{–34}\ \mathrm{J} \bullet \mathrm{s}
{"title":"Energy of a photon","description":"Wrong","type":"incorrect","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"poly\",\"coords\":\"144,22\"},{\"shape\":\"rect\",\"coords\":\"1,7,41,53\"}]"} {"title":"Wave frequency","description":"Incorrect","type":"incorrect","color":"#ffff00","code":"[{\"shape\":\"rect\",\"coords\":\"134,5,160,62\"}]"} {"title":"Planck’s constant = 6.62606957 × 10 sup –34 J • s","description":"Correct!","type":"correct","color":"#00ff00","code":"[{\"shape\":\"rect\",\"coords\":\"103,5,133,54\"}]"}Review
To see how it’s possible to violate energy conservation, let’s consider the photon. We learned in Section 22-4 that the energy of a photon is proportional to its frequency:
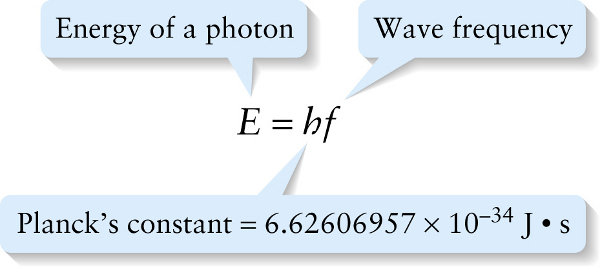
For a photon to have a definite energy, it must therefore have a definite frequency. However, a wave \psi with a definite frequency also has an infinite duration (Figure 28 5a). This means that wave has always been present and will always be present. A more realistic description of a wave is one that has a finite duration: for example, the wave produced when you turn a source of waves (like a laser pointer) on and then off again. Mathematically, a wave of a finite duration \Delta{t} can be expressed as a sum of waves of infinite duration like the one shown in Figure 28-5a, but with a range of frequencies of breadth \Delta{f} (Figure 28-5b). To make a shorter-duration wave, we have to add together infinite-duration waves from a broader range of frequencies (Figure 28-5c).