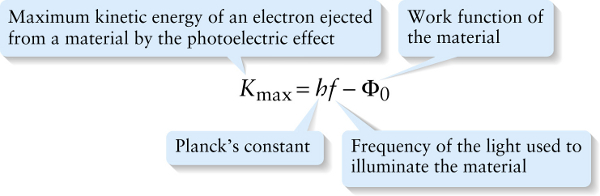
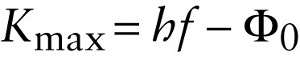Maximum kinetic energy of an electron in the photoelectric effect (26-1)
Question 1 of 4
Question
Frequency of the light used to illuminate the material
{"title":"Maximum kinetic energy of an electron ejected from a material by the photoelectric effect","description":"Wrong","type":"incorrect","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"poly\",\"coords\":\"144,22\"},{\"shape\":\"rect\",\"coords\":\"1,2,35,45\"}]"} {"title":"Work function of the material","description":"Incorrect","type":"incorrect","color":"#ffff00","code":"[{\"shape\":\"rect\",\"coords\":\"244,6,279,47\"}]"} {"title":"Frequency of the light used to illuminate the material","description":"Correct!","type":"correct","color":"#00ff00","code":"[{\"shape\":\"rect\",\"coords\":\"177,2,201,56\"}]"} {"title":"Planck’s constant","description":"Incorrect","type":"incorrect","color":"#ff0000","code":"[{\"shape\":\"rect\",\"coords\":\"149,4,177,51\"}]"}Review
Equation 26-1 tells us that that a graph of Kmax as a function of the light frequency f should be a straight line of slope h (see Figure 26-3). Since kinetic energy can never be negative, Equation 26-1 also tells us that electrons will be emitted only if hf−Φ0>0.