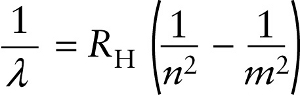Rydberg formula for the spectral lines of hydrogen (26-14)
Question 1 of 3
Question
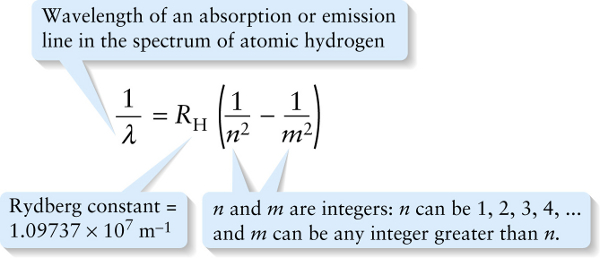
Wavelength of an absorption or emission line in the spectrum of atomic hydrogen
{"title":"Wavelength of an absorption or emission line in the spectrum of atomic hydrogen","description":"Correct!","type":"correct","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"poly\",\"coords\":\"144,22\"},{\"shape\":\"rect\",\"coords\":\"5,55,29,91\"}]"} {"title":"n and m are integers: n can be 1, 2, 3, 4, ... and m can be any integer greater than n.","description":"Incorrect","type":"incorrect","color":"#ffff00","code":"[{\"shape\":\"rect\",\"coords\":\"162,61,184,88\"},{\"shape\":\"rect\",\"coords\":\"243,64,276,88\"}]"} {"title":"Rydberg constant = 1.09737 × 10 sup 7 m sup −1","description":"Incorrect","type":"incorrect","color":"#00ff00","code":"[{\"shape\":\"rect\",\"coords\":\"86,22,113,62\"}]"}Review
The value of the constant RH in Equation 26-14, called the Rydberg constant, is chosen to match the experimental data. To four significant figures, RH=1.097×107 m−1 As an example, the hydrogen absorption and emission lines shown in Figure 26-12 all correspond to n=2 in Equation 26-14. The series of wavelengths for which n=2 are called the Balmer series.