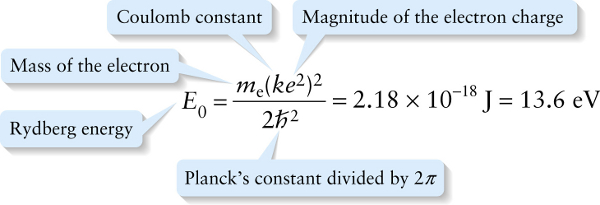
Rydberg energy (26-26)
Question 1 of 5
Question
Magnitude of the electron charge
{"title":"Rydberg energy","description":"Wrong","type":"incorrect","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"poly\",\"coords\":\"144,22\"},{\"shape\":\"rect\",\"coords\":\"1,18,12,33\"}]"} {"title":"Mass of the electron","description":"Incorrect","type":"incorrect","color":"#ffff00","code":"[{\"shape\":\"rect\",\"coords\":\"37,10,54,20\"}]"} {"title":"Coulomb constant","description":"Wrong","type":"incorrect","color":"#00ff00","code":"[{\"shape\":\"rect\",\"coords\":\"65,5,75,21\"}]"} {"title":"Magnitude of the electron charge","description":"Correct!","type":"correct","color":"#ff0000","code":"[{\"shape\":\"rect\",\"coords\":\"73,10,86,22\"}]"} {"title":"Planck’s constant divided by 2 pi","description":"Incorrect","type":"incorrect","color":"#333333","code":"[{\"shape\":\"rect\",\"coords\":\"66,31,77,48\"}]"}Review
In Equation 26-26 we’ve given the value of the Rydberg energy in joules and in electron volts (eV). The energy that an electron acquires when it moves through a potential difference of 1 V is 1 eV, or approximately 1.602×10−19 J. To give you an idea of the amount of energy 1 eV represents, a photon of visible light carries between about 1.5 and 3 eV.