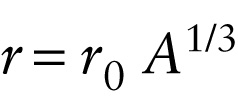Radius of a nucleus (27-1)
Question 1 of 3
Question
Radius of a nucleus
{"title":"Radius of a nucleus","description":"Correct!","type":"correct","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"poly\",\"coords\":\"144,22\"},{\"shape\":\"rect\",\"coords\":\"1,41,27,80\"}]"} {"title":"Mass number of the nucleus (total number of protons and neutrons)","description":"Incorrect","type":"incorrect","color":"#ffff00","code":"[{\"shape\":\"rect\",\"coords\":\"142,30,181,79\"}]"} {"title":"r sub 0 = 1.2 ± 0.2 fm","description":"Incorrect","type":"incorrect","color":"#00ff00","code":"[{\"shape\":\"rect\",\"coords\":\"79,41,105,77\"}]"}Review
The size of a nucleus (its radius and volume) is related to the mass number A. Experiments show that all nuclei are approximately spherical and have radii proportional to the cube root of A, or A1/3:
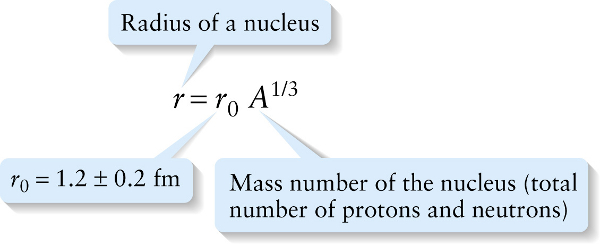
This equation states that if we increase the number of nucleons in a nucleus by a factor of 10, say from A=20 to A=200, the radius of the nucleus will increase by a factor of 101/3=2.2 (Figure 27-3).