Binding energy of a nucleus (27-2)
Question 1 of 7
Question
Speed of light in a vacuum
{"title":"Binding energy of a nucleus","description":"Wrong","type":"incorrect","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"poly\",\"coords\":\"144,22\"},{\"shape\":\"rect\",\"coords\":\"2,12,17,31\"}]"} {"title":"Number of neutrons in the nucleus","description":"Incorrect","type":"incorrect","color":"#ffff00","code":"[{\"shape\":\"rect\",\"coords\":\"56,11,77,33\"}]"} {"title":"Number of protons in the nucleus","description":"Wrong","type":"incorrect","color":"#00ff00","code":"[{\"shape\":\"rect\",\"coords\":\"130,12,148,32\"}]"} {"title":"Mass of a neutral atom (including electrons) containing that nucleus","description":"Wrong","type":"incorrect","color":"#ff0000","code":"[{\"shape\":\"rect\",\"coords\":\"214,14,237,32\"}]"} {"title":"Speed of light in a vacuum","description":"Correct!","type":"correct","color":"#333333","code":"[{\"shape\":\"rect\",\"coords\":\"281,15,294,33\"}]"} {"title":"Mass of a neutral hydrogen atom (1 proton + 1 electron","description":"Incorrect","type":"incorrect","color":"#ff6600","code":"[{\"shape\":\"rect\",\"coords\":\"148,16,169,32\"}]"} {"title":"Mass of a neutron","description":"Incorrect","type":"incorrect","color":"#0000ff","code":"[{\"shape\":\"rect\",\"coords\":\"76,15,97,31\"}]"}Review
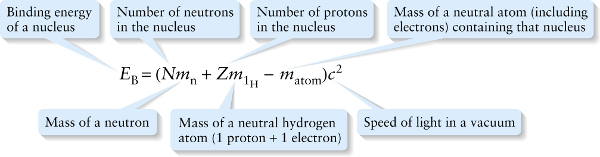
To find the binding energy of 4He, we subtracted the mass of the nucleus from the mass of the two protons and two neutrons separately, and then multiplied by c2 to find the equivalent energy. In general, for a nucleus consisting of N neutrons and Zprotons, EB is
EB=(Nmn+Zmp−mnucleus)c2
where mn is the mass of a neutron, mp is the mass of a proton, and mnucleus is the mass of the nucleus. In practice, it’s easier to measure the masses of neutral atoms (including their electrons) than the masses of isolated atomic nuclei. In terms of these masses, we can write the binding energy of a nucleus as