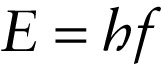Energy of a photon (22-26)
Question 1 of 3
Question
Wave frequency
{"title":"Energy of a photon","description":"Wrong","type":"incorrect","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"poly\",\"coords\":\"144,22\"},{\"shape\":\"rect\",\"coords\":\"1,8,41,55\"}]"} {"title":"Wave frequency","description":"Correct!","type":"correct","color":"#ffff00","code":"[{\"shape\":\"rect\",\"coords\":\"135,4,161,63\"}]"} {"title":"Planck’s constant = 6.62606957 × 10–34 J • s","description":"Incorrect","type":"incorrect","color":"#00ff00","code":"[{\"shape\":\"rect\",\"coords\":\"102,4,133,59\"}]"}Review
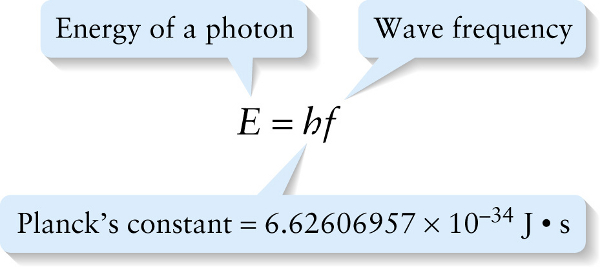
In 1905, the same year that he published his special theory of relativity, Albert Einstein proposed a simple but radical explanation for the strange behavior of the photoelectric effect. He suggested that light of frequency f comes in small packets, each with an energy E that is directly proportional to the frequency. Today these packets are called photons. We first encountered this idea in Section 22-4: