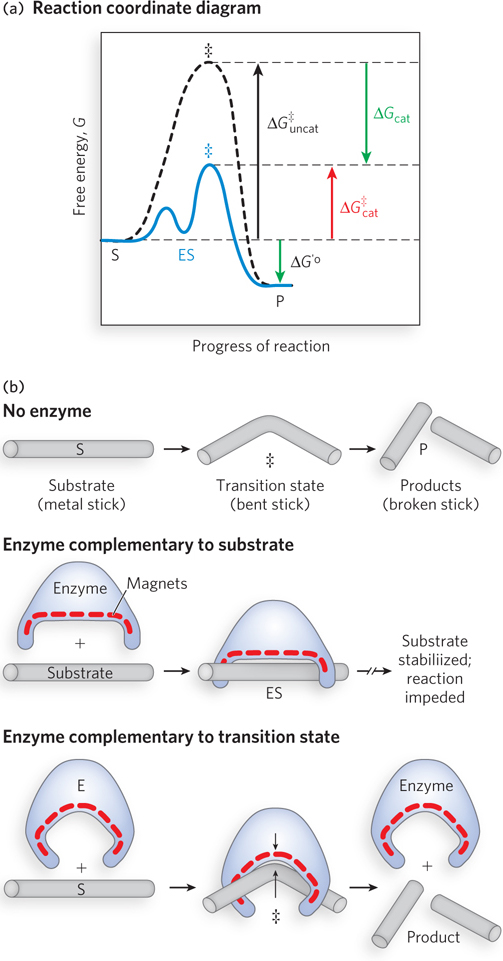
The use of noncovalent binding energy to accelerate an enzyme- catalyzed reaction. (a) A reaction coordinate diagram. The free energy of a system is plotted against the progress of the reaction S → P. This kind of diagram describes the energy changes during the reaction; the horizontal axis (reaction coordinate) reflects the progressive chemical changes (e.g., bond breakage or formation) as S is converted to P. The activation energies, ΔG‡, for the S → P and P → S reactions are indicated. ΔG′° is the overall biochemical standard free- energy change in the direction S → P. The ES intermediate occupies a minimum in the energy progress curve of the enzyme- catalyzed reaction. The terms ΔG‡uncat and ΔG‡cat correspond to the activation energy for the uncatalyzed reaction (black, dashed curve) and the overall activation energy for the catalyzed reaction (blue, solid curve), respectively. The activation energy is lowered by the amount ΔGcat when the enzyme catalyzes the reaction. (b) An imaginary enzyme (stickase) designed to catalyze the breaking of a metal stick. Before the stick is broken, it must be bent (transition state). Magnetic interactions replace weak enzyme- substrate bonding interactions. A stickase with a magnet- lined pocket that is structurally complementary to the stick (substrate) stabilizes the substrate (middle). Bending is impeded by the magnetic attraction between stick and stickase. An enzyme with a pocket complementary to the reaction transition state helps destabilize the stick (bottom), contributing to catalysis. The binding energy of the magnetic interactions compensates for the increase in free energy needed to bend the stick. In enzyme active sites, weak interactions that occur only in the transition state aid in catalysis.