5.3 Motor Proteins
Organisms move. Cells move. Organelles and macromolecules within cells move. Most of these movements arise from the activity of motor proteins, a fascinating class of protein-based molecular motors. Fueled by chemical energy, usually derived from ATP, organized groups of motor proteins undergo cyclic conformational changes that create a unified, directional force—the tiny force that pulls apart chromosomes in a dividing cell and the immense force that levers a quarter-ton jungle cat into the air.
As for all proteins, interactions among different motor proteins, or between motor proteins and other types of proteins, include complementary arrangements of ionic bonds, hydrogen bonds, hydrophobic interactions, and van der Waals interactions at protein-binding sites. In motor proteins, however, these interactions achieve exceptionally high levels of spatial and temporal organization.
Motor proteins promote the contraction of muscles (actin and myosin), the migration of organelles along microtubules (kinesin and dynein), the rotation of bacterial flagella, and the movement of some proteins along DNA. In molecular biology, the motor proteins of most interest are those that function in the transactions involving nucleic acids. These include the helicases, proteins that unwind double stranded DNA or RNA; DNA and RNA polymerases that synthesize polynucleotide chains; DNA topoisomerases that relieve the tension created by overwound or underwound DNA; and other proteins that move along DNA as they carry out their functions in DNA metabolism. These motor proteins are described in detail in Chapters 9–16. Here, we focus on helicases, motor proteins that are highly relevant to the information pathways explored in this text. A great many human genetic diseases have been traced to defects in motor proteins of this class, attesting to their general importance.
Motor proteins combine the functions of ligand binding and catalysis. Each motor protein must interact transiently with another macromolecular ligand, binding and releasing it in a reversible process. To bring about productive motion, the sequence of binding and release cannot be random; it must have a unidirectional component. This requires energy, usually derived from ATP hydrolysis, which is coupled to a directed bind-and-release process between protein and ligand. For the motor proteins we are concerned with, the ligand is generally DNA or RNA.
Helicases Abound in DNA and RNA Metabolism
A helicase is a protein that separates the paired strands of a nucleic acid, converting a duplex into two single strands. Helicases are part of a larger family of motor proteins that promote reactions by translocating along the DNA (or RNA) substrate, resulting in the displacement of proteins from nucleic acids, the separation of DNA strands that is required for replication and recombination, conformational changes in nucleic acids, and the remodeling of chromatin. All of these processes are coupled to the hydrolysis of ATP; enzymes, including helicases, that hydrolyze ATP are often referred to as ATPases.
Two structural classes of ATPase domain are found in helicases. The first is structurally related to the core domain of the bacterial RecA recombinase (see Chapter 13); ATP is often bound in a site near the intersection of two RecA-like domains (Figure 5-13a). The second is related to a class of enzymes called AAA+ (ATPases associated with various cellular activities); again, ATP is bound in sites located at the subunit-subunit interfaces (Figure 5-13b).
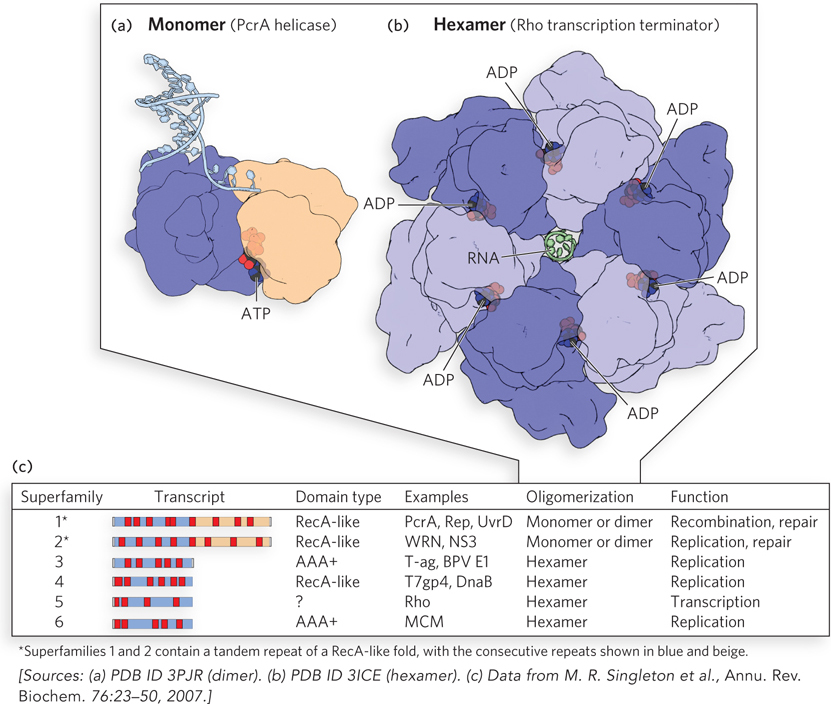
Figure 5-13: DNA helicases. (a) Enzymes of helicase superfamilies 1 and 2 (SF1 and SF2) have a conserved core structure, the RecA-like fold. Here, an ATP analog (black and red) that mimics ATP structure but is not hydrolyzed is bound at the interface of the two domains in a monomeric helicase PcrA. The use of such ATP analogs is often necessary to obtain a structure that illustrates the complex. (b) The core of helicases of SF3 through SF6 consists of six subunits with nucleotide-binding pockets at the subunit-subunit interfaces. Shown here is the Rho transcription termination factor (discussed in Chapter 15). In the structure shown, ADP was present in the crystallization mixture, and its bound location in the resulting structure indicates the ATPase active site. (c) Helicase superfamilies. Core domains (blue and beige) and positions of the conserved sequence motifs that characterize a protein as a helicase (red) are shown for each family (“Transcript”). The sequence motifs are universal structural elements in all helicases and include motifs involved in the binding and hydrolysis of a nucleoside triphosphate. There are two different ATPase domain types in helicases, as shown in the “Domain type” column. Well-studied examples of each family, typical oligomeric structure, and functions are listed in the remaining three columns.
Based on extensive sequence comparisons, six superfamilies of helicases have been defined (Figure 5-13c). Most of them include proteins that do not function in DNA or RNA strand separation but instead are involved in translocation along DNA or RNA. Helicases of superfamilies 1 and 2 (SF1 and SF2), the most common, usually have two RecA-like domains. These proteins often, but not always, function best as oligomers. Helicases of superfamilies 3 through 6 (SF3–SF6) generally function as circular hexamers (see Figure 5-13b and this chapter’s Moment of Discovery). The individual domains are RecA-like in superfamily 4. The subunits in SF3 and SF6 helicases have the AAA+ structural domain.
The superfamilies are further defined on the basis of a series of amino acid sequence motifs (see Figure 5-13c). At least three motifs found in all helicase superfamilies are involved in ATP binding and hydrolysis. Other motifs are involved in DNA or RNA binding or oligomerization.
Helicase Mechanisms Have Characteristic Molecular Parameters
Any discussion of helicase mechanisms must take into account several key biochemical properties of these motor proteins: oligomeric state, rate of nucleic acid unwinding or translocation, directionality, processivity, step size, and ATP-coupling stoichiometry.
For some helicases, the oligomeric state in which they are active has been quite controversial. A few helicases exhibit some activity as monomers but are greatly stimulated by the addition of more subunits or auxiliary proteins. The observed rates of DNA unwinding or translocation can thus be a complicated function of reaction conditions and proteins added.
Helicases are associated with DNA unwinding, the separation of the two paired strands of a nucleic acid. Closely related proteins are often involved in translocation, which is movement along duplex DNA or RNA without separating the paired strands. These latter proteins are sometimes called translocases, but many other names are used that are more closely attuned to a protein’s specific function. In some cases, these proteins are bound to a structure such as a membrane or a viral coat and function to pump DNA or RNA through it. Others function to eject bound proteins from a nucleic acid.
Helicases move unidirectionally on a strand of nucleic acid, and directionality—the direction in which the enzyme moves along the strand—is an important distinguishing characteristic of a helicase mechanism. Some helicases move only in the 3′→5′ direction, and some only in the 5′→3′ direction (Figure 5-14). With double-stranded DNA, the direction of movement is defined by only one strand. The strand that guides the direction of movement is established during the process of loading the helicase onto the DNA. Helicases that translocate in the 3′→5′ direction seem to be more common than those moving in the 5′→3′ direction.
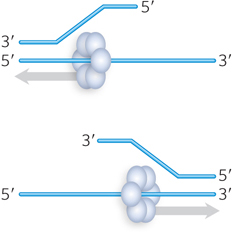
Figure 5-14: Direction of movement by helicases. Helicases can be classified based on translocation directionality: 3′→5′ (top) or 5′→3′ (bottom).
Processivity refers to the number of base pairs unwound or the number of nucleotides translocated, on average, each time an enzyme of this type binds to a DNA molecule. Some helicases may unwind only a few base pairs before dissociating, whereas a much greater processivity is the norm for hexameric helicases that encircle DNA and function in such processes as chromosomal DNA replication.
The step size is the average number of base pairs or nucleotides over which the helicase moves for each ATP molecule hydrolyzed. The ATP-coupling stoichiometry is the average number of ATP molecules consumed per base pair or nucleotide traversed. This may seem like another way of describing step size, and the two can be closely related. However, if coupling is not perfect and some ATP is hydrolyzed unproductively, the step size measured experimentally can be lower than the true step size that reflects the coupled ATP hydrolysis–movement cycle.
Most helicases seem to use a translocation mechanism that can be described as “stepping.” It requires the helicase to have at least two DNA-binding sites. Using a series of conformational changes facilitated by ATP binding and hydrolysis, the enzyme moves along the DNA. Different sites on the same or different helicase subunits bind alternately to the DNA strand defining directionality, in a closely orchestrated reaction sequence (Figure 5-15). When multiple subunits are employed (dimers or hexamers), DNA molecules may get passed between the subunits and step sizes can be larger.
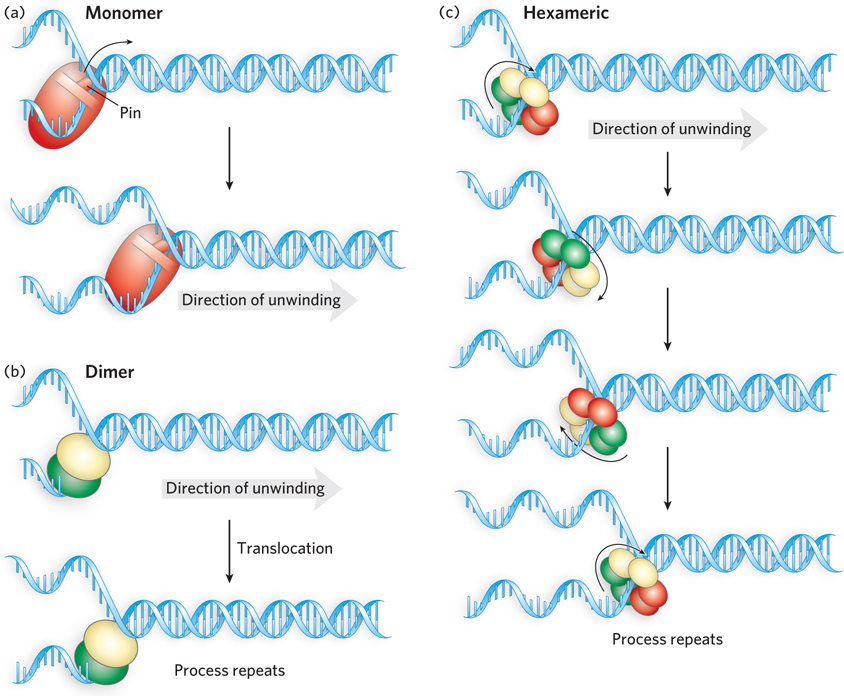
Figure 5-15: Unwinding reaction mechanisms for DNA helicases. (a) A monomeric helicase hydrolyzes ATP, causing conformational changes that couple unidirectional DNA translocation with destabilization of the duplex. Some of these enzymes have pinlike structures that function in strand separation. (b) A functional dimer model. A dimeric helicase has two identical subunits. These may alternate in binding to single- and double-stranded DNA or may translocate linearly along one strand of DNA (as shown here). In either case, the protein unwinds the DNA as it moves. (c) Hexameric helicases move along one DNA strand of a duplex DNA, separating the strands as it moves. The bound DNA strand is usually bound alternately by three pairs of dimers within the hexameric structure. The passing of the strand from one dimer to another is coupled to conformational changes driven by ATP hydrolysis. In the diagram, the red, green, and yellow subunit pairs represent the three states: one state has bound ATP, the second has bound ADP (immediately after hydrolysis), and the third has no bound nucleotide.
Helicase translocation is necessary but not sufficient for the unwinding of a nucleic acid. Unwinding mechanisms are classified as active or passive. In an active mechanism, the enzyme interacts directly with double-stranded DNA or RNA to destabilize the duplex structure. In a passive mechanism, the enzyme interacts only with single strands, moving onto the strands and stabilizing them as they form through normal thermal base-pair opening and closing. Although active mechanisms are not yet well understood, they seem to be used by many helicases. In many cases, the destabilization of a double-stranded DNA or RNA is facilitated by a structure often described as a pin, a kind of wedge that pries the two strands apart. The ATPase motor pushes or pulls the paired strands past the pin (as seen in Figure 5-15a). Some dimeric helicases may employ an unwinding mechanism in which one subunit interacts with and destabilizes the duplex DNA, while the other subunit acts as a translocase on adjacent single-stranded DNA (see Figure 5-15b).
A great variety of motor proteins, many of them in the helicase families, carry out a range of functions other than nucleic acid unwinding. We will encounter many examples in later chapters, and we consider just a few here.
The RuvB protein (SF6 class; its name derives from resistance to UV irradiation) is a DNA translocase involved in the movement of four-armed DNA junctions called Holliday intermediates that appear during DNA recombination (we discuss recombination and Holliday intermediates in Chapter 13). Acting with the RuvA protein, two hexamers of RuvB are arranged symmetrically to propel the DNA, catalyzing a rapid migration of the DNA branch to which they are bound (Figure 5-16a).
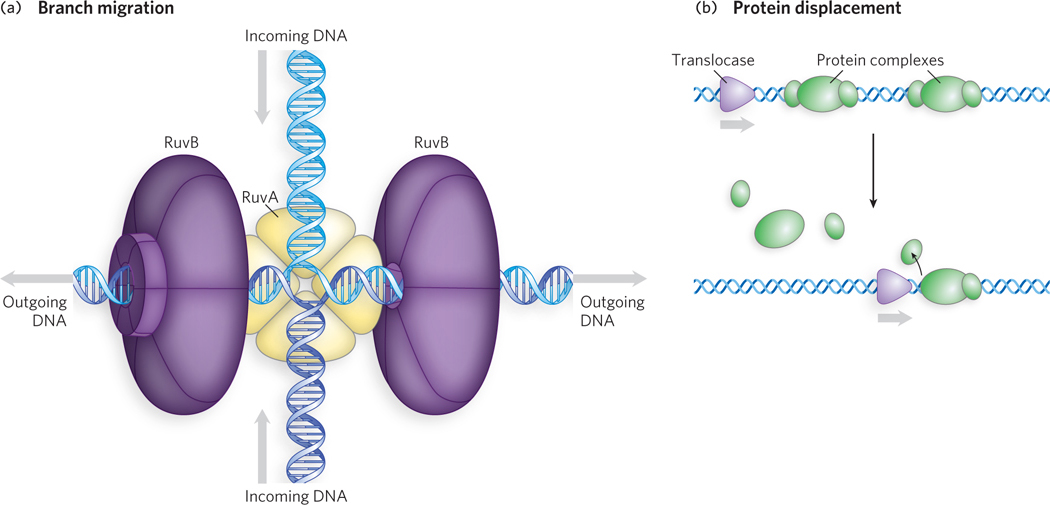
Figure 5-16: DNA translocases. (a) Branch migration during double-stranded DNA translocation. The bacterial RuvA and RuvB proteins form a complex that binds to a four-armed DNA junction that often forms during recombination (a Holliday intermediate; see Chapter 13), and the RuvA protein binds to the junction itself. The RuvB protein is the translocase, propelling the DNA to the left and right, as shown (arrows). (b) Protein displacement during DNA translocation. Translocase proteins in both bacteria and eukaryotes displace proteins from nucleic acids.
The eukaryotic Snf proteins (SF2 class; named for sucrose nonfermenting) are involved in the alteration and/or disruption of a variety of protein-DNA interactions. Each of the many Snf proteins has a particular target protein or protein complex, or set of targets, including RNA polymerase, nucleosomes, and regulatory DNA-binding proteins. Snf proteins interact directly with their targets, utilizing their motor functions to displace or reposition the target proteins on the DNA (Figure 5-16b). Snf proteins are also heavily involved in chromatin remodeling (see Chapter 10). The NPH-II RNA helicase (SF2 class; NTP (nucleoside triphosphate) phosphohydrolase) is a viral helicase that unwinds RNA, but it is also very effective as a translocase that displaces proteins from single-stranded RNA. SF2 enzymes are particularly common and important in RNA metabolism. Many are critical to the maturation of large ribonucleoprotein complexes, such as ribosomes and spliceosomes.
With respect to ATP hydrolysis, motor proteins generally exhibit the standard steady-state kinetic behavior of an enzyme. An example is the Michaelis-Menten kinetics seen for ATP hydrolysis by the helicase PcrA in the presence of single-stranded DNA (Figure 5-17). PcrA is an SF1 class helicase found in gram-positive bacteria.
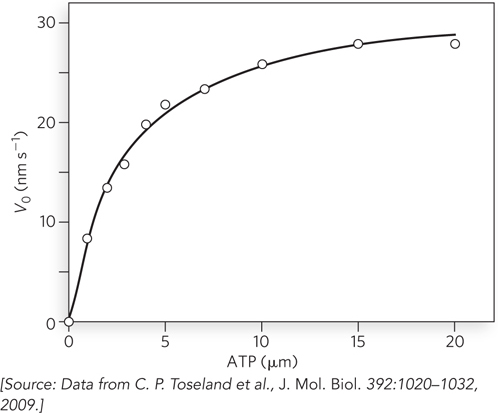
Figure 5-17: A plot of V0 versus [S] for the hydrolysis of ATP by the helicase PcrA. This hydrolytic reaction follows classic Michaelis-Menten kinetics. Compare this with Figure 5-11b.
SECTION 5.3 SUMMARY
Motor proteins combine the functions of ligand binding and catalysis. The ligand, often RNA or DNA, is bound reversibly. Movement involves protein conformational changes triggered by ATP hydrolysis catalyzed by the motor protein.
Helicases are ubiquitous motor proteins involved in DNA or RNA strand separation. Helicase mechanisms are discussed in terms of oligomeric state, rate of nucleic acid unwinding or translocation, directionality, processivity, step size, and ATP-coupling stoichiometry.
Many motor proteins closely related to helicases are involved in translocation along nucleic acids, movement of branches in double-stranded DNA during replication, protein displacement from nucleic acids, chromatin remodeling, and other functions.




