Nematode, Caenorhabditis elegans
Small single-celled microbes such as E. coli and yeast are overwhelmingly successful models for studying the fundamental chemistry of life processes at the cellular level, but multicellular models are needed to investigate the complexities of development and the nervous system. In the 1960s, Sidney Brenner recognized that it was time to ask questions about the nervous system and how a multicellular organism develops from a single cell. Brenner decided on the nematode worm, a small, translucent metazoan, as a model for pursuing these new questions.
Caenorhabditis elegans is a member of the Rhabditidae family of nematodes, but unlike other family members, it is not pathogenic or parasitic to other animals. C. elegans is an appropriate choice as a model for animal development because it grows rapidly and, depending on temperature, can develop from an egg to a sexually mature adult in 2½ to 5½ days. Easy to grow on agar plates with E. coli as a food source, the worms can even be frozen for long-term storage. C. elegans hermaphrodites can self-fertilize, a property that allows quick recovery of homozygous mutants from a population of mutagenized worms. Males, produced by self-fertilizing hermaphrodites at a low frequency, can mate with hermaphrodites to create strains with new mutant combinations, an essential aspect of any genetic model organism. In addition, C. elegans is transparent, allowing every cell to be visualized during development. Although the hermaphrodite contains only 959 somatic cells, the nematode is highly complex, with a nervous system, muscles, and digestive and reproductive systems, and exhibits a variety of behaviors for neurological genetic studies. Each worm produces hundreds of progeny, allowing mutant screens for all types of anatomic and behavioral changes.
Early Studies of C. elegans as a Model Organism
To lay a foundation for studies on how a multicellular organism develops, Brenner and others mapped the location of all 959 cells in the hermaphrodite worm by image reconstruction of tissue slices. During development, each migratory cell travels to a precise position in the animal. Further studies led researchers to the discovery that about 12% of cells consistently die during development and that cell death is genetically programmed. We now know that programmed cell death is important to the development of higher organisms, including humans, and that defects in this process can lead to cancer.
A prime example of the use of C. elegans as a model for development is studies on the vulva. Development of this 22-cell organ has been studied intensively, as its individual cells are easily visualized in the microscope. Screens to identify defects in vulval development exploit the fact that the vulva is a nonessential organ, and when disrupted, development produces an easily recognized phenotype. Fertilized eggs in the uterus must be deposited through the vulva to hatch outside the body. With developmental defects in the vulva, eggs cannot be deposited and they hatch internally. The microscope reveals many worms inside the mother (sometimes referred to as a “bag of worms”). Using this obvious mutant phenotype, researchers have identified numerous genes that control vulval development, including genes in a conserved phosphorylation pathway that controls cell growth. Mammalian homologs of some of these genes encode tumor suppressors and oncogenes.
Programmed cell death in C. elegans development results in the death of one of the two progeny cells following cell division at various stages of development. This mechanism is essential for the proper developmental path of the surviving progeny cell. Many examples of programmed cell death are documented in C. elegans, several of which occur during development of the nervous system. Programmed cell death is also important in human embryonic development, such as in the removal of tissue between the fingers and toes.
There are two sexes in C. elegans, hermaphrodite and male. Hermaphrodites contain two copies of the X chromosome. A low percentage of hermaphrodite germ cells (0.05% to 1.0%) undergo nondisjunction of the X chromosomes during meiosis, to produce so-called null-X gametes. When a null-X gamete fertilizes a normal (X) gamete, the resulting zygote has only one X chromosome (X0) and develops as a male. Hermaphrodites produce 250 to 300 self-sperm, limiting self-progeny to 250 to 300 embryos; the fertilized eggs follow a multistage life cycle. Hermaphrodites crossed with a male preferentially use the male’s sperm for fertilization and can produce hundreds of additional cross-progeny embryos that, other than the sex ratio, are identical to self-progeny and follow a multistage life cycle (Figure A-4). After embryogenesis, which takes about 12 hours at 25°C, eggs hatch to produce larvae 80 μm long. The initial larva contains 558 cells. Development proceeds through four larval stages (L1 through L4) separated by molts. The final molt results in the sexually competent adult worm. The overall process takes about 52 hours at 25°C. Adults live for approximately 15 days. Under stressful conditions, L2 larvae may enter a dormant stage, the dauer larva, which can persist for several months, waiting for conditions to improve.
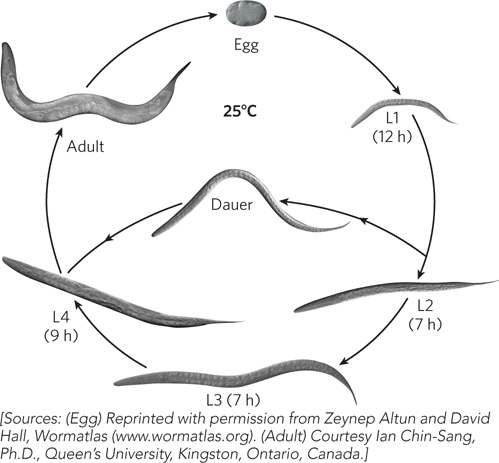
Figure A-4: The life cycle of Caenorhabditis elegans.
Mutagenesis Worms can be mutagenized with chemical treatment or irradiation. Mutant worms can be screened for phenotypic alterations by direct observation with the microscope. Phenotypes include aberrant behavior or development, the inability of certain cells to undergo programmed cell death, altered life span, and inability of larvae to enter the dauer stage.
Self-Fertilization and Cross-Fertilization Self-fertilization produces about 250 to 300 progeny from one hermaphrodite and allows rapid recovery of recessive mutant alleles from individual mutant worms. Although males are produced only rarely, they provide opportunities to construct new genetic stocks by crossing with a hermaphrodite.
Introduction of DNA In transformation studies, linear recombinant DNA is injected directly into the gonad before the eggs are formed. Injected DNA from long tandem arrays that behave like artificial chromosomes is inefficiently transmitted through the germ line. This DNA can be induced to integrate into the genome by gamma irradiation. Rare integration events lead to stable inheritance of transgene arrays. Integration rarely occurs by homologous recombination.
Gene Knockouts Disruption by a transposon was a common method to destroy the function of a C. elegans gene. Transposon mutagenesis is rarely performed any more. It has been replaced by random mutagenesis and whole genome sequencing or, more recently, by site-directed cleavage and nonhomologous end joining to create deletions or homologous recombination to engineer mutants and transgenes into the genome.
RNA Interference RNA interference, originally discovered in the nematode, can be used to silence gene function by introducing double-stranded RNA homologous to the gene under study (see Chapter 22).
C. elegans as a Model Organism Today
Signaling Pathways Programmed cell death and vulval development use signaling pathways that are apt models for human signaling pathways. Studies of C. elegans development continue to elucidate important features of these processes and related pathways.
Human Disease C. elegans has many genes that are homologous to human disease genes, including those in the insulin-signaling pathway, as well as genes involved in heart, kidney, and neurological diseases. Study of these disease genes may illuminate the basis for human diseases.
Aging Genetic studies of the dauer larva have identified a set of genes that, when switched on in the adult, dramatically extend the worm’s life span. The presence of homologs of these genes in other animals has obvious implications for the study of aging.
RNA-Based Control of Gene Expression Studies of RNA interference, first discovered in the nematode system, have identified miRNAs involved in gene expression. Indeed, miRNAs are now known to be involved in gene regulation in all plants and animals. The sensitivity of worms to ingested dsRNA (environmental RNAi) enables rapid, whole genome screens for genes important in specific processes.
Neurodevelopment The C. elegans nervous system is the animal’s most complex organ, comprising over one-third of its cells (302 neurons and 56 glial cells). Unlike the highly branched neuronal connections of vertebrates, the connectivity of neurons in C. elegans is relatively simple, with about 5,000 chemical synapses and 2,000 neuromuscular junctions. Behavioral abnormalities are easily observed and can be mapped with precision to particular neuronal networks. Knowledge of the complete neuronal connectivity enables researchers to study how axon growth is guided and how synapses form. In addition, C. elegans has several different classes of neurons, enabling genetic studies of neuronal differentiation.

