House Mouse, Mus musculus

The house mouse, Mus musculus, has been collected and bred by mouse “fanciers” for hundreds of years, and some of the purebreds developed by fanciers are used today as standards in scientific studies. The house mouse is the leading mammalian model, and it is more like a human than we may care to admit (see Figure 1-12). The mouse genome is almost the size of the human genome and encodes essentially the same number of genes, 99% of which have homologs in the human. In fact, much of the mouse’s genome is syntenic with ours, meaning that whole blocks of genes occur in the same order in both species (see Figure 8-4).
Compared with other model organisms, mice are more cumbersome to work with in every way. They are larger, of course, but they also have a generation time of about 8 to 10 weeks and produce, on average, only 3 to 14 pups per litter. These statistics are attractive when compared with other mammals, but pale in comparison with other model organisms. Colonies of mice are also costly to maintain, and they simply cannot be dealt with in the numbers needed to perform large genetic screens, as with other model organisms. However, unlike the nematode and fruit fly, mice have biological systems that have no parallel in lower animal models, such as the immune and skeletal systems, or are simply better models for studies of complex systems such as the cardiovascular system, endocrine system, and many others. The mouse is a model for human disease, including cancer, in virtually all of these systems.
Early Studies of the Mouse as a Model Organism
Genetic studies in mice began in the early 1900s, when selection and breeding were the main methods of obtaining progeny with the desired traits. These early studies produced a general model that explained coat coloring in all other fur-
Life Cycle
The X and Y chromosomes determine sex in mice, as in humans. Fertilization gives rise to a blastocyst containing some undifferentiated cells bunched together in the inner cell mass, the source of embryonic stem cells. Gestation is complete within 19 to 21 days and gives rise to a litter of 3 to 14 pups. Sexual maturity requires about 6 weeks for females and 8 weeks for males, but breeding can take place in as short a time as 35 days. Mice live for 1 to 2 years, and females can produce about 5 to 10 litters, mainly in their first year of life.
Genetic Techniques
Mutagenesis Inbreeding over many generations has produced many useful strains of mutant mice. Adding mutagenic chemicals to the food supply also facilitates development of mutant strains.
Introduction of DNA Foreign DNA can be injected directly into the nucleus of fertilized eggs, followed by implantation of the eggs in the oviduct of the female recipient. Random integration occurs with high frequency. The recombinant DNA used typically has a mouse promoter that directs expression of a reporter gene, such as lacZ or GFP (green fluorescent protein), so that expression of the transgene can be followed during development. About half of the transgenic mice contain recombinant DNA in the germ line and therefore pass on the recombinant gene to future generations.
Gene Knockouts Targeted knockouts for mouse disease models are constructed in embryonic stem cells (Figure A-
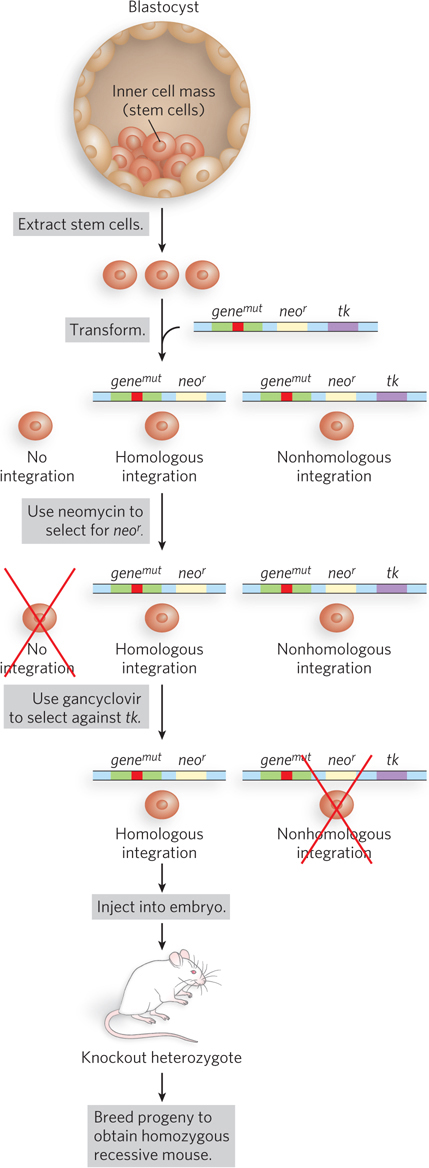
A-
To select for cells with the desired gene knockout, two steps are required. Selection for neor, using neomycin, kills all cells that fail to integrate transformed DNA. Selection against tk, using the antiviral gancyclovir, kills cells with DNA integrated by nonhomologous recombination, because these cells contain tk and thymidine kinase converts gancyclovir to a toxin that kills these cells. Only cells that contain gene knockouts produced by homologous recombination survive both selections. Engineered stem cells are injected into a host wild-
More recently, flippase–
The Mouse as a Model Organism Today
Human Disease The mouse is an important model for studying human disease and aging. Disease models can be derived by inbreeding or by producing knockouts of known disease genes. Mouse models of human disease include cancer, atherosclerosis, hypertension, metabolic diseases, immune disorders, type 1 and type 2 diabetes, Down syndrome, Alzheimer disease, glaucoma, osteoporosis, obesity, epilepsy, Lou Gehrig disease (amyotrophic lateral sclerosis), Huntington disease, blood disorders, and many others.
Mapping of Mutant Genes Mutant genes can be identified more quickly in the mouse than can mutant genes in humans. Closely related strains of mice (e.g., Mus musculus and Mus spretus) can be crossed to produce hybrids that usually contain different sequences at polymorphic positions, enabling researchers to use linkage analysis to develop detailed genetic maps and locate a disease gene.
Behavior Mouse models exist for certain types of behavior, including alcoholism, drug addiction, anxiety disorders, and aggressive behavior.
Mammalian Development Transgenic mice are used to study the location and timing of expression of particular genes at various stages of development. In addition, models exist for studying certain human developmental disorders, including cleft lip and cleft palate.
A-