12.2 DNA ALTERATIONS THAT LEAD TO MUTATIONS
DNA is subject to damage by a variety of sources. Inside the cell, reactive chemical species generated in normal metabolic processes can damage DNA. One might not immediately think of water as a DNA-damaging agent, yet water—the universal intracellular solvent—takes its toll on DNA through spontaneous hydrolysis reactions that damage nucleotides and the DNA backbone. Also, the high degree of negative charge on DNA makes it prone to electrophilic attack by alkylating agents and by reactive oxygen species (such as hydrogen peroxide, hydroxyl radicals, and superoxide radicals), many of which are present in the normal intracellular environment. DNA is also susceptible to damage from external sources. Various types of radiation, including x rays and UV light, can cause chemical changes in DNA. Chemicals, both natural and synthetic, can directly damage DNA or are metabolized to DNA-damaging agents. We discuss here some of the most common types of DNA damage caused by these sources.
Each of these agents is capable of causing a particular type of damage to DNA. Hydrolytic damage and alkylating agents can affect the phosphodiester backbone or a nucleotide base. Irradiation can cause slightly larger lesions, such as cross-links between bases or breaks in the DNA strand. And the DNA sequence can even be altered by the very enzymes responsible for its preservation. In fact, some of the errors occurring during replication and recombination can be among the most difficult for the cell to detect and sometimes escape cellular mechanisms of repair.
Spontaneous DNA Damage by Water Can Cause Point Mutations
Hydrolysis is the cleavage of a molecule by the addition of water. The reactive hydroxide ion (HO−) is formed when a proton (H+) dissociates from water (see Chapter 3). Hydrolysis reactions are often initiated by nucleophilic attack by a hydroxide ion; the leaving group acquires the proton, giving a net addition of water to the substrate (Figure 12-8a). At physiological pH, the concentration of hydroxide ion is low, so hydrolysis is slow—otherwise, life as we know it could not exist. However, given the large number of nucleotides in a genome, damage to nucleotide bases by even a slow rate of hydrolysis quickly adds up and becomes significant to the cell.
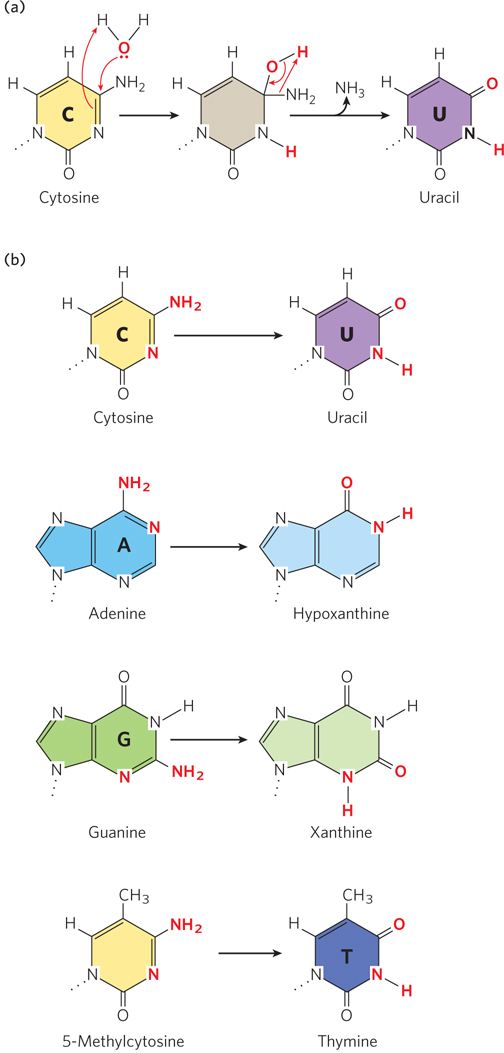
Figure 12-8: Deamination of nucleotide bases by spontaneous hydrolysis. In these deamination reactions, only the base is shown for each nucleotide residue. (a) A hydrolysis reaction in which water is added to cytosine, resulting in deamination to uracil and ammonia. A similar mechanism occurs in other deaminations. (b) Common deamination reactions resulting from hydrolysis of nucleotides in DNA.
One result of hydrolysis is the deamination of nucleotide bases (see Figure 12-8a). Deamination is the removal of an amino group from a compound, and all molecules that contain an amino group are possible targets of hydrolytic attack. Three of the nucleotide bases contain an amino group—cytosine, adenine, and guanine—and thus can be deaminated by hydrolysis (Figure 12-8b). Deamination of cytosine is the most common, and it results in uracil (see Chapter 6). Adenine and guanine also undergo deamination, but at only one-hundredth the rate of cytosine deamination. Deamination of adenine and guanine, unlike that of cytosine, produces bases not normally found in nucleotides: adenine yields hypoxanthine, which pairs with cytosine if left uncorrected; guanine forms xanthine, which is less deleterious because it still pairs with cytosine, through two hydrogen bonds instead of three.
Left unrepaired, a C → U change would be highly mutagenic, because uracil pairs with adenine more readily than with guanine. Hence, if the change is not repaired before replication, the cell will replicate the U to form a U=A base pair without pausing, replacing the original C≡G base pair. Repair of the uracil after replication would then insert thymine in place of uracil, completing the C≡G to T=A transition mutation. However, because DNA contains thymine rather than uracil, uracil in DNA is readily recognized as foreign. A biological need to avoid mutations during long-term maintenance of genomic DNA may have driven the evolution of the use of T in place of U in DNA so that the spontaneous appearance of U might be readily repaired.
In higher eukaryotic cells, in about 5% of C residues in DNA the cytosine is methylated to 5-methylcytosine (forming 5-methylcytidine, 5-meC), a modification that is linked to gene expression. Methylation is most common on C residues that are followed by a G, in CpG sequences. Methylation at CpG sequences produces 5-meCpG symmetrically on both strands of the DNA. Deamination of 5-methylcytosine produces thymine instead of uracil (see Figure 12-8b). Because thymine is a naturally occurring base in DNA, the modification is not recognized as damage and has the potential to become a transition mutation in which a G≡C base pair is changed to an A=T base pair. However, the damage does result in a mispair, from a 5-meC–G to a T–G base pair, and can be recognized by the cell’s mismatch repair system (described below) before the next round of replication. Nevertheless, positions of 5-meC residues in eukaryotic DNA are associated with mutational hotspots.
Another relatively frequent hydrolytic reaction is the attack of water on the N-β-glycosyl bond between the base and the pentose of a nucleotide residue, breaking the connection between the base and the DNA backbone. This type of hydrolytic reaction leaves an abasic site, a position in an intact DNA backbone that is without a base. Hydrolysis of the N-β-glycosyl bond occurs at a higher rate for purines—a process referred to as depurination—than for pyrimidines (Figure 12-9). In fact, as many as 1 in 105 purines (10,000 per mammalian cell) are lost from DNA every 24 hours. Depurination can lead to a mutation when the abasic site forms in single-stranded DNA, because the information in the complementary strand is not present and the DNA polymerase often inserts an incorrect nucleotide during replication to form the duplex DNA. Base loss also results in spontaneous ring opening in the deoxyribose moiety, causing a weakening of the DNA backbone that can lead to strand breaks.
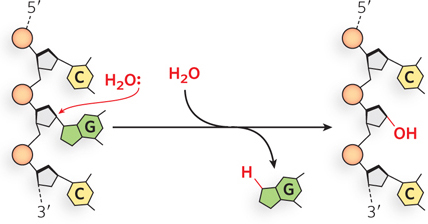
Figure 12-9: Depurination resulting from hydrolysis. In depurination, a purine (in this case, guanine) is lost from DNA by hydrolysis of the N-β-glycosyl bond.
Oxidative Damage and Alkylating Agents Can Create Point Mutations and Strand Breaks
The highly negatively charged DNA molecule is susceptible to electrophilic attack by alkylating agents and reactive oxygen species. Some of these DNA-damaging agents, such as cigarette smoke and industrial pollut ants, come from the external environment. Often, synthetic and natural chemicals that damage DNA are not reactive per se but are transformed into DNA-damaging chemicals by modification reactions. Another plentiful source of DNA-damaging reagents is reactive oxygen generated inside cells by aerobic metabolism (i.e., by the electron transfer chain in mitochondria) and by the detoxification system in the liver. All cells contain enzymes that convert reactive oxygen species into harmless molecules, but some of these reactive species escape the cellular cleansing systems and can damage DNA and other biomolecules.
Nitrous Acid–Induced Deamination Environmental pollutants that are metabolized to DNA-reactive forms include sodium nitrate (NaNO3), a common food preservative, which is converted in the stomach to nitrous acid (HNO2). Nitrosamines and nitrate salts are also converted to nitrous acid. Nitrous acid acts as a mutagen by reacting with A and C residues, altering their ability to form correct base pairs (Figure 12-10). If the modified bases are not repaired, a transition mutation will result. Bisulfite (HSO3−) has similar effects and is also used as a food preservative. Sodium nitrate and bisulfite do not seem to increase the risk of cancer in humans when used in this way, perhaps because, in such small amounts, they make only a minor contribution to the overall level of DNA damage.
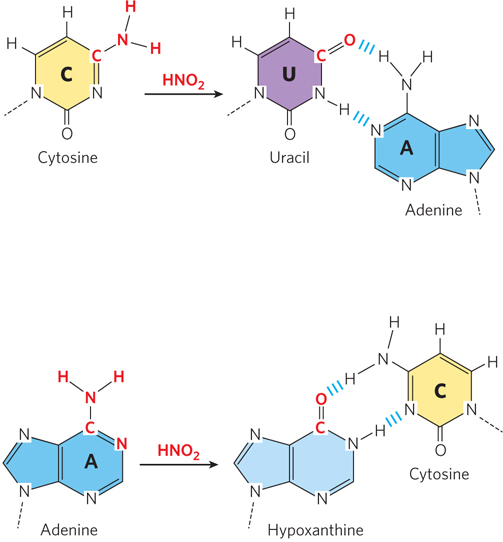
Figure 12-10: Deamination by nitrous acid. Nitrous acid can deaminate C and A residues, causing them to base-pair with the wrong nucleotide during replication. The incorrect base pairing after deamination is shown on the right.
Oxidative Damage Possibly the most important source of mutagenic alterations in DNA is oxidative damage. The DNA of each cell in the body is subjected to thousands of damaging oxidative reactions every day. Reactive oxygen species, such as hydrogen peroxide (H2O2), hydroxyl radicals (·OH−), and superoxide radicals (·O2−), arise during irradiation or as byproducts of aerobic metabolism. Oxidative DNA damage ranges from oxidation of the base to oxidation of the deoxyribose sugar to the removal of bases (forming abasic sites), and it can also cause strand breaks.
The hydroxyl radical reacts with both purine and pyrimidine bases. In pyrimidines, the double bond between C-5 and C-6 is highly susceptible to attack, resulting in a variety of oxidized bases, including 5-hydroxyuracil, 5-hydroxycytosine, uracil glycol, and thymine glycol (Figure 12-11a). In purines, too, oxidation generates a spectrum of products. Among these reactions is the oxidation of guanine to 8-oxoguanine, which is extremely mutagenic. In the syn conformation (see Figure 6-16), an 8-oxoG residue base-pairs with A, and replication that occurs before 8-oxoG is repaired results in a G≡C to T=A transversion mutation (Figure 12-11b). Such G≡C to T=A transversions are among the most common mutations in human cancers.
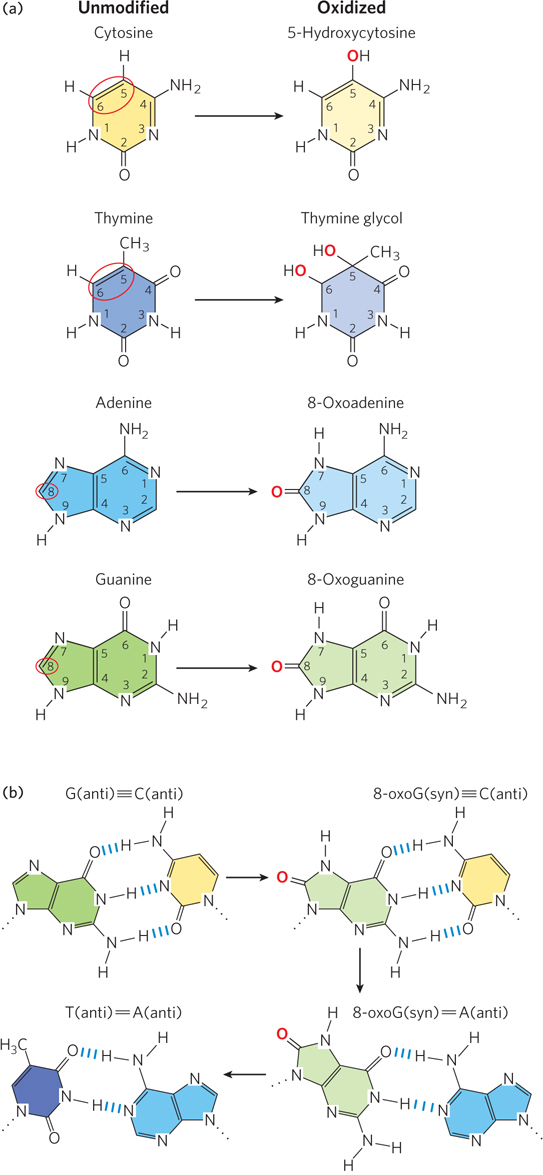
Figure 12-11: Oxidative damage to purine and pyrimidine bases. (a) Common positions (circled in red) of oxidative damage to nucleotide bases (left) and the products of oxidation at these positions (right). (b) Oxidation of the guanine of a G residue to 8-oxoguanine is extremely mutagenic. The 8-oxoG residue base-pairs with either A or C and thus can result in a G≡C to T=A transversion.
Damage by Alkylation The addition of an alkyl group to atoms in nucleotide bases or to the phosphodiester backbone is known as alkylation. The N-3 of adenine and O6 of guanine are common sites of alkylation, although many other positions in all four nucleotide bases, as well as the phosphodiester backbone of DNA, can also be modified, depending on the alkylating agent (Figure 12-12a). Alkylation of a nucleotide base can have a range of effects on base pairing, from no effect to completely preventing base pairing of the alkylated base with another base.
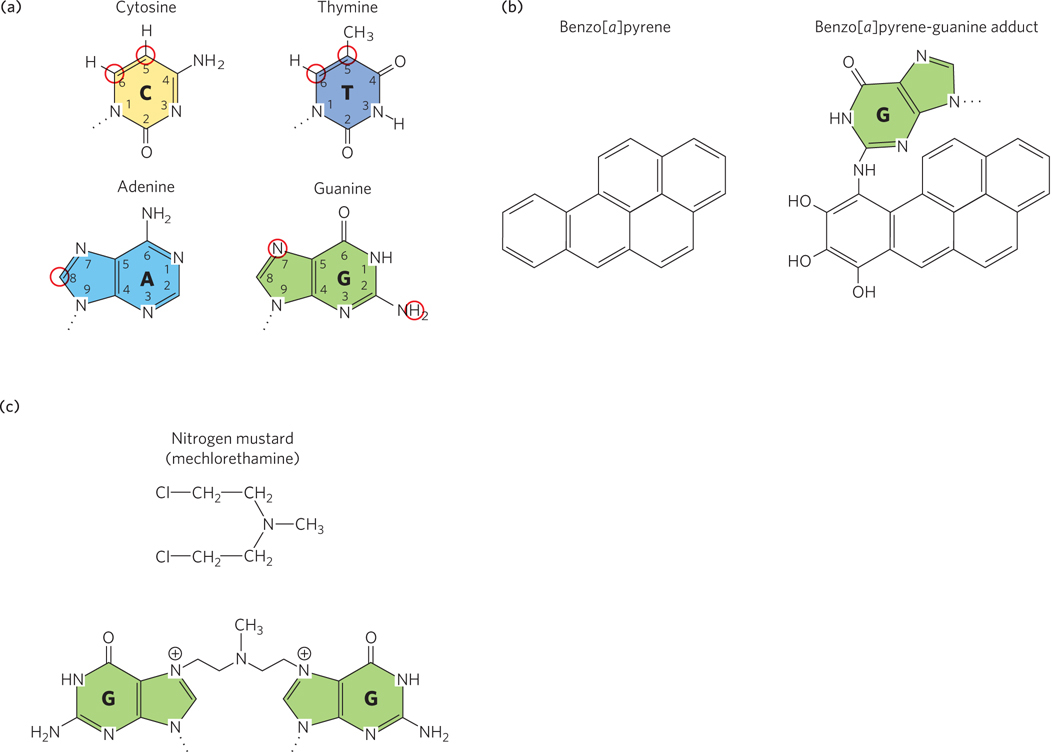
Figure 12-12: Alkylation of DNA. Only the bases of the nucleotide residues are shown. (a) Atoms that are most susceptible to alkylation (circled in red). (b) Benzo[a]pyrene is hydroxylated in the liver to a reactive form (an epoxide) that alkylates nucleotide bases; the product of its reaction with guanine is shown. (c) A nitrogen mustard forms interstrand cross-links between two G residues.
Any substance directly involved in promoting cancer is called a carcinogen. A known carcinogen in the smoke of burning cigarettes, wood, and coal tar that reacts with DNA is benzo[a]pyrene. Benzo[a]pyrene is an intercalating agent and is in a class of chemicals referred to as polycyclic aromatic hydrocarbons, which are hydroxylated in the liver as part of a detoxification process. However, hydroxylation of some polycyclic aromatic hydrocarbons results in a highly reactive epoxide. In the case of benzo[a]pyrene, hydroxylation forms an epoxide that reacts with purines: with N6 of adenine or N2 of guanine (Figure 12-12b). Although N2 of guanine is not a base-pairing atom, these bulky alkylated bases no longer form correct base pairs and can lead to mutations if not corrected.
Nitrogen mustard gas is an alkylating agent that was used as a weapon in World War I. Nitrogen mustards are cross-linking agents, and they react with adjacent G residues to form interbase cross-links (Figure 12-12c).
The Ames Test Identifies DNA-Damaging Chemicals
DNA-reactive compounds are referred to as genotoxic, because they cause chemical changes in genomic DNA. Many sources of cancer in humans can be traced to DNA-damaging agents. In a seeming contradiction, not only can DNA-damaging agents cause cancer, but some are used in chemotherapy to treat some types of cancer. Small amounts of a mutagen can do just enough damage to cause cancer. But large amounts can do more than cause mutations: they can kill the cell—they are cytotoxic—and can sometimes be used to kill cancer cells.

Bruce Ames
It clearly is in our interest to identify carcinogenic substances so that we can choose to avoid them and/or put them to use in treating cancer. Many mutagens are also carcinogens. A rapid and inexpensive test for a chemical mutagen is provided by a bacterial screen invented by Bruce Ames. The Ames test makes use of a strain of Salmonella typhimurium that contains a mutation in the biosynthetic pathway for histidine and therefore requires histidine in the growth medium. These mutants are called auxotrophs, which are cells that have lost the capacity to synthesize various organic compounds such as amino acids. Histidine-auxotrophic S. typhimurium cells plated on a medium lacking histidine do not survive. But a small number of the cells may acquire mutations that reverse the original mutation, and cells with a reversion mutation (or back mutation) of this type can synthesize histidine. These mutant cells can survive on a histidine-free medium and form a small number of colonies that grow in random positions when the cell culture is grown on a petri plate (Figure 12-13a).
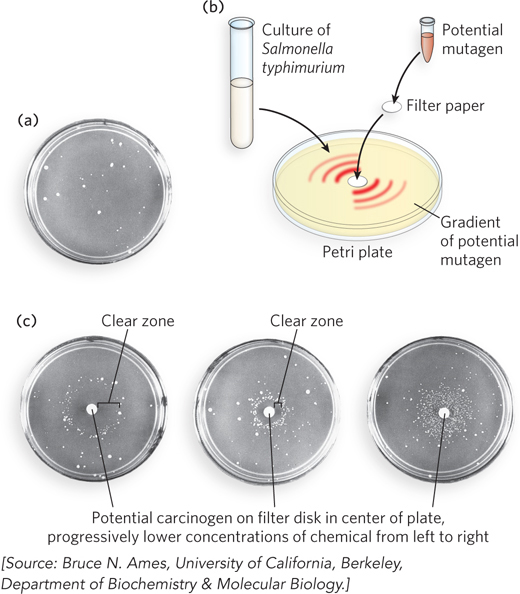
Figure 12-13: The Ames test for carcinogens, based on mutagenicity. The Ames test uses a strain of Salmonella typhimurium lacking an enzyme needed to synthesize histidine; the bacterium is plated on a histidine-free medium. (a) In the absence of the chemical to be tested, a few cells spontaneously develop a reversion mutation and form colonies. (b) The chemical to be tested is soaked into a filter-paper disk placed in the center of the plate, and the chemical diffuses to create a concentration gradient in the plate. (c) Identical nutrient plates are inoculated with an equal number of cells, but have progressively lower concentrations of the putative mutagen on the filter-paper disk (from left to right). At lower concentrations, the clear zone around the disk is smaller because fewer cells are killed by the chemical.
To test a potential mutagen (and thus a potential carcinogen), the compound is absorbed on a filter-paper disk placed in the center of a plate that contains S. typhimurium in the growth medium (Figure 12-13b). The chemical diffuses from the disk into the medium, creating a concentration gradient. The clear zone immediately adjacent to the disk contains the highest concentration of compound, too high to support life (Figure 12-13c). Beyond this lethal zone, the mutagen produces reversion mutations in some cells that enable S. typhimurium to form colonies. Because some compounds are mutagens only after they have been hydroxylated in the liver, the Ames test includes a step in which the compound being tested is first incubated with a liver extract. Most known human carcinogens result in increased mutation in the Ames test. In fact, numerous common industrial chemicals and natural products produce increased mutation in this test. The Ames test is therefore only the start of a process that identifies a compound as a human carcinogen. Compounds identified as mutagens in an Ames test require further testing in animals to determine whether they are likely to be human carcinogens.
DNA-Damaging Agents Are Used in Cancer Chemotherapy
DNA-reactive agents used in chemotherapy for cancer kill cells by creating broken chromosomes or stalled replication forks, either of which leads to cell death during cell division. The cytotoxic effect of DNA-damaging agents therefore requires the cell to be actively dividing. Chemotherapeutic agents are toxic to cancer cells because these cells must divide to form a tumor, but not toxic to most somatic cells that are not dividing. Nondividing or slowly dividing cells do sustain DNA damage during chemotherapy, but the damage is often repaired before replication occurs. The adverse side effects of chemotherapeutic DNA-damaging agents (hair loss, anemia, and nausea) are due largely to their effects on the few cell types in the body that rapidly divide, such as hair follicle cells, blood cells, and the cells that line the digestive tract.
Some types of DNA-damaging agents are particularly efficient at blocking replication forks or breaking chromosomes. A common and potent chemotherapeutic agent is the cross-linking drug cisplatin. Cisplatin is an alkylating agent that forms covalent adducts with the N-7 position of the two purine residues (Figure 12-14a). The intrastrand and interstrand cross-links resulting from reaction with cisplatin can be hard to repair, and they persist until they are encountered by a replication fork, leading to arrest of replication and cell death. Cisplatin is used in the treatment of many cancers, including bone cancer, lung cancer, certain lymphomas, and ovarian cancer.
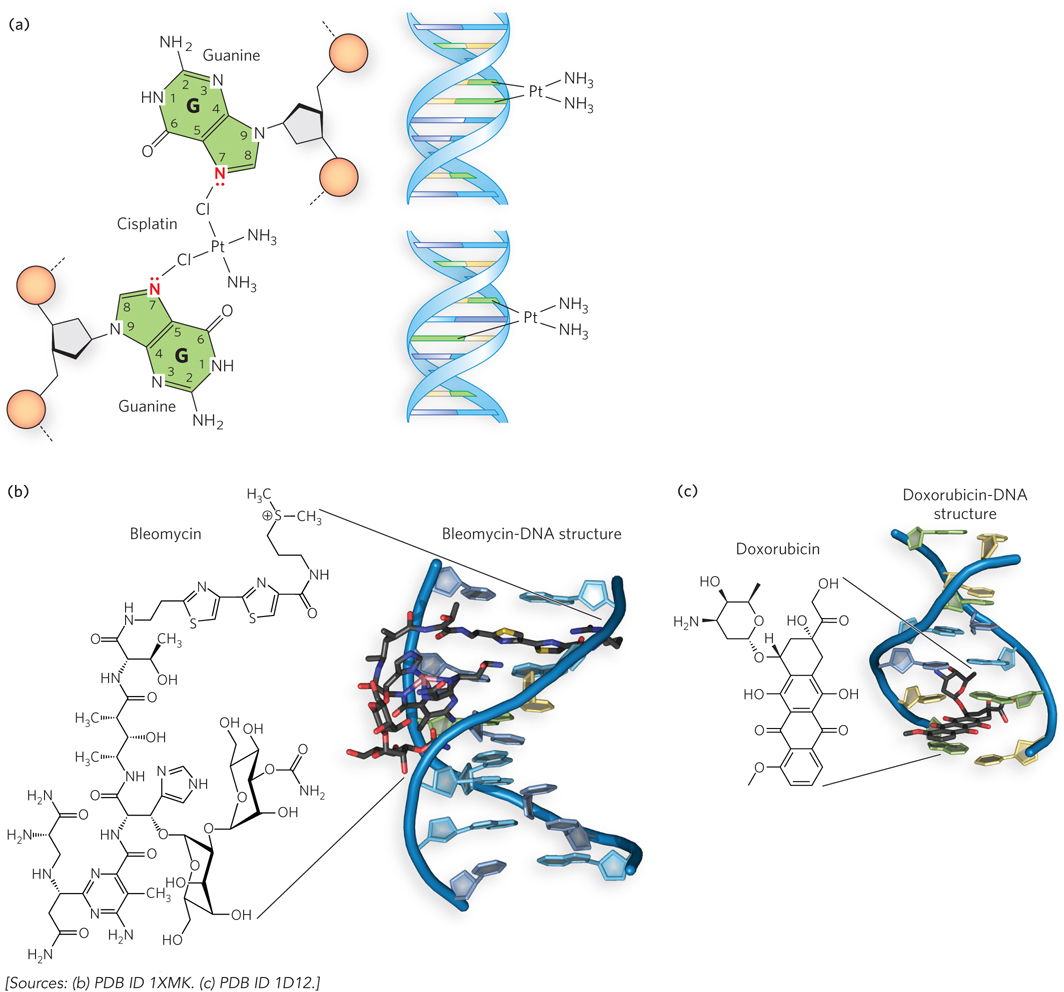
Figure 12-14: Chemotherapeutic DNA-damaging agents. Chemotherapeutic agents act by preferentially damaging the DNA of fast-dividing tumor cells. (a) Cisplatin is a cross-linking reagent that reacts with N-7 of two G residues to form intrastrand or interstrand cross-links. (b) Bleomycin binds to DNA and forms reactive oxygen species (not shown) that cause strand breaks. Iron normally binds bleomycin, but cobalt was used to prevent DNA modification in the crystal structure shown here. (c) Doxorubicin is a DNA intercalator, inserting between adjacent nucleotide residues. Intercalators can cause frameshift mutations during replication, or they can block replication forks, making the forks susceptible to nucleases that cause strand breaks.
Bleomycin, a complex biomolecule isolated from a bacterium, binds an atom of iron and activates molecular oxygen to form hydroxyl radicals that damage DNA. Bleomycin also binds to DNA, and its proximity to DNA directs the hydroxyl radical–mediated damage (Figure 12-14b). Bleomycin is used to treat Hodgkin lymphoma and testicular cancer.
Anthracyclines are chemotherapeutic agents that function by intercalating into DNA, reversibly inserting between base pairs. Doxorubicin is one such agent, and intercalation leads to double-strand breaks by inhibiting the resealing step of topoisomerase II (Figure 12-14c). Doxorubicin is used to treat leukemias, Hodgkin lymphoma, and several other types of cancer, including ovarian and breast cancer and tumors of the stomach, bladder, and thyroid gland.
Chemotherapeutic DNA-damaging agents are frequently used in combination and kill cells by blocking replication or transcription, often by triggering programmed cell death. Unfortunately, the damage incurred by chemotherapy also leads to mutations in some normal cells, and people who survive the primary cancer are at increased risk of developing a secondary tumor later in life. Therapies that more precisely target cancer cells alone are under development.
Solar Radiation Causes Interbase Cross-Links and Strand Breaks
Virtually all forms of life are exposed to energy-rich radiation that can cause chemical changes in DNA. We are subject to a constant field of ionizing radiation in the form of UV light (in sunlight) and cosmic rays, which can penetrate deep into the Earth, as well as radiation emitted from radioactive elements such as radium, plutonium, uranium, radon, carbon-14, and tritium (3H). X rays used in medical and dental examinations, and in radiation therapy to treat cancer and other diseases, are other sources of exposure. UV and other ionizing radiation is estimated to be responsible for about 10% of all DNA damage caused by environmental agents.
Nucleotide bases interact very strongly with UV light (wavelengths of 200 to 400 nm), which can promote reactions that chemically change the DNA. Pyrimidine dimers are formed by UV-induced covalent cross-links between neighboring pyrimidines on the same DNA strand. Pyrimidine dimers form through the condensation of two ethylene groups on adjacent pyrimidines to form a cyclobutane ring (Figure 12-15a, left). Adjacent T residues or adjacent C residues can react to form a 6-4 photoproduct (see Figure 12-15a, right). Pyrimidine dimers cause a significant distortion in the DNA and thus can no longer base-pair with another nucleotide (Figure 12-15b). On encountering a pyrimidine dimer during replication, the DNA polymerase stalls; repair is therefore a matter of urgency.
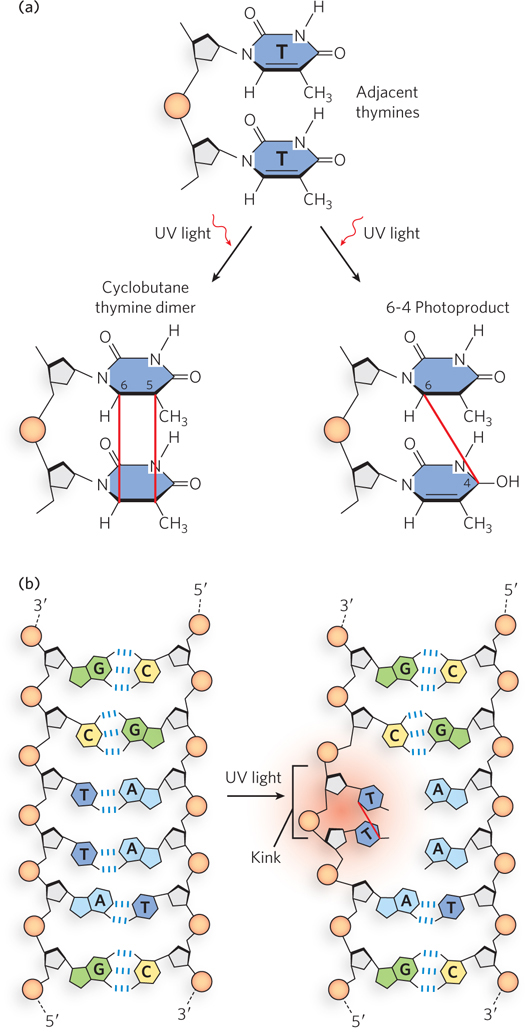
Figure 12-15: Pyrimidine dimers and their effect on the DNA duplex. (a) One type of reaction caused by UV light results in a cyclobutane ring, which involves C-5 and C-6 of adjacent pyrimidine (in this case, thymine) bases. An alternative reaction results in a 6-4 photoproduct that links C-6 and C-4 of adjacent pyrimidines. (b) Formation of a pyrimidine dimer introduces a bend or kink in the DNA.
Gamma rays and x rays are much higher-energy radiation than UV light and generate reactive oxygen species that can break one or both strands of DNA (Figure 12-16). A break in a single strand of duplex DNA is easily repaired because it can be rejoined by ligase. But when both strands are broken, the repair job is much more difficult. Rejoining of a broken chromosome is performed by either homologous recombination or nonhomologous end-joining reactions, as we de scribe in detail in Chapter 13. These repair reactions, especially the latter, often result in mutations due to the loss of nucleotide bases.
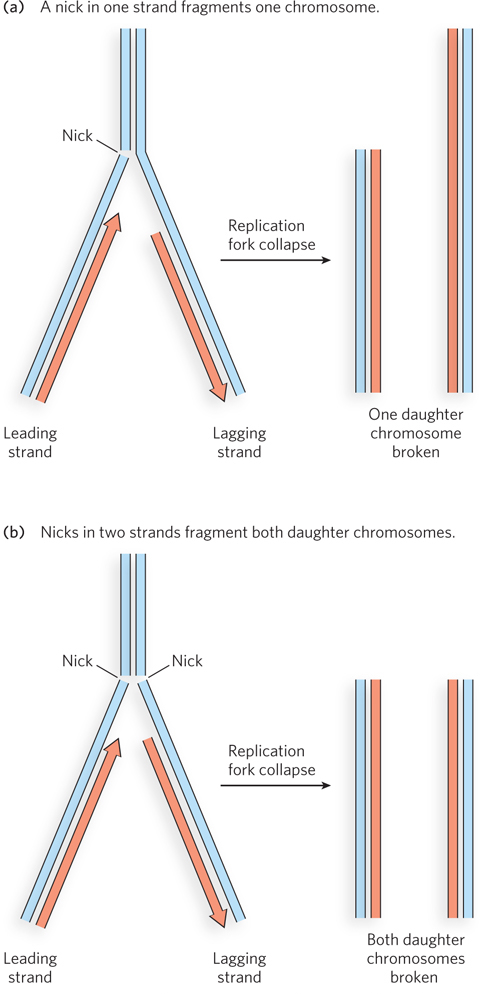
Figure 12-16: DNA damage caused by strand breaks during replication. (a) When a replication fork encounters a single-strand break (nick), one daughter chromosome is broken, while the other daughter chromosome remains intact. The intact chromosome can become a template for repair of the broken one through homologous recombination (described in Chapter 13). (b) When the break occurs in both strands, both daughter chromosomes are broken, and neither daughter chromosome is completed.
Errant Replication and Recombination Lead to DNA Damage
Although it seems counterintuitive, many types of mutations arise from the activity of the very proteins that have evolved to maintain the integrity of genomic DNA. For example, DNA polymerases can cause damage. As we learned in Chapter 11, DNA polymerases are highly accurate and have a very low probability of making a mistake. But given the large size of genomes, even a low probability becomes a certainty. Sometimes, tautomers of nucleotide bases form correct matches with the DNA template and lead to base-pair mismatches, and sometimes the proofreading 3′→5′ exonuclease misses an incorrect base, and a mismatch results. Also, the polymerase can slip on repetitive sequences (template slippage), giving rise to insertion or deletion mutations. Recall, too, that not all DNA polymerases are as accurate as the primary replicase. There are rare instances in which the replication fork encounters a damaged base and low-fidelity translesion DNA polymerases are recruited that can insert an incorrect nucleotide in order to move the replication fork past the damage—allowing replication to continue, but leading to a mutation.
During cell division, homologous chromosomes are lined up in pairs so that they can be partitioned into daughter cells. The proximity of similar DNA sequences can lead to the exchange of DNA segments through homologous recombination during meiosis or mitosis. Errors in this process sometimes occur. For example, a repeated nucleotide sequence on a chromosome can lead to recombination between two regions on homologous chromosomes or between two nonhomologous chromosomes. This can lead to chromosomal aberrations such as deletions, duplications, inversions, insertions, and translocations.
When chromosomal abnormalities arise during development, the anomaly is present in every cell in the body that is descended from the mutant cell. Chromosomal abnormalities can also arise in a somatic cell of an adult, and when this happens, the abnormalities are present only in a subset of cells, such as those in a tumor formed from the mutated cell.
SECTION 12.2 SUMMARY
Hydrolysis can deaminate nucleotide bases, altering their ability to base-pair and leading to a mismatch during replication. Deamination of cytosine to uracil is the most common type and, if not repaired, can lead to a C≡G to T=A transition mutation. Hydrolysis can also sever the glycosyl bond between the pentose and the base, leaving an abasic site.
Nitrous acid, the metabolic product of a food preservative, can induce the deamination of A or C residues, resulting in a transition mutation.
Oxidative damage is caused by reactive oxygen species that react with nucleotides at many different positions in the molecule. Oxidation can affect base pairing or cause DNA strand breaks.
Alkylating agents attack DNA at any of several electron-rich atoms, adding bulky chemical groups to the base or the phosphodiester backbone. Alkylation can alter the base pairing of the nucleotide.
DNA-damaging agents can lead to mutations at low concentrations and can kill the cell at high concentrations. The Ames test determines whether a compound is mutagenic in bacteria, thus identifying the compound as a potential carcinogen. Chemotherapy for cancer patients often uses DNA-damaging agents at high concentrations, thereby killing cancer cells that are replicating faster than most normal cells.
UV light from the sun can form pyrimidine dimers that stall DNA polymerase during replication. X rays and gamma rays cause single-strand and double-strand DNA breaks.
DNA damage can also result from errant replication, which can produce point mutations or small insertions and deletions. Errant recombination can result in large-scale chromosomal abnormalities.









