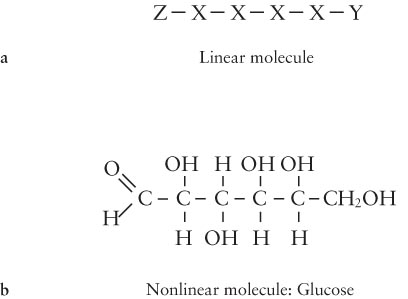
Figure 15- 2 Creating Complex Molecules (a) Atoms (denoted by capital letters) that can bond strongly to only two other atoms can make linear chains, as depicted here. However, when such atoms bond to atoms that can only make one bond, denoted here as Y and Z, the chain stops. In no case can atoms X, Y, and Z combine to create nonlinear chains other than loops. (b) When an atom, like carbon (C), can share electrons with more than two other atoms (in carbon’s case, with four atoms), then the complex, nonlinear chains essential for life can form. Chains of carbon atoms form the backbone of organic molecules. For example, glucose, with carbon, oxygen (O), and hydrogen (H), is a nonlinear molecule that serves as a nutrient for many life- forms; it is a sugar. The lines indicate bonds between atoms.