THE NATURE OF LIGHT
So far in this text we have used the word light in its everyday sense—the stuff to which our eyes are sensitive. This is more properly called visible light, and it is a form of electromagnetic radiation. Perhaps contrary to one’s intuition, this radiation is composed of particles, called photons, that have properties of waves. Detecting electromagnetic radiation with telescopes is the essence of observational astronomy. Although human perception of objects here on Earth and in space comes primarily from the visible light that our eyes detect, visible light is only a tiny fraction of all the electromagnetic radiation emitted by objects in the universe. The rest of this radiation, invisible to our eyes, is detected by high-tech sensors attached to specially designed telescopes. In this chapter, we examine telescopes for all types of electromagnetic radiation. To understand how telescopes work, we begin by exploring the properties of the electromagnetic radiation that they collect.
3-1 Newton discovered that white is not a fundamental color and proposed that light is composed of particles
From the time of Aristotle (in the fourth century b.c.e.) until the late seventeenth century c.e., most people believed that white is the fundamental color of light. The colors of the rainbow (or, equivalently, the colors created by light passing through a prism) were believed to be added or created somehow as white light went from one medium through another. Isaac Newton performed experiments during the late 1600s that disproved these beliefs. He started by passing a beam of sunlight through a glass prism, which spread the light out into the colors of the rainbow (Figure 3-1a). The change in direction as light travels from one medium into another is called refraction, and the resulting spread of colors (complete or with colors missing) is called a spectrum (plural, spectra).
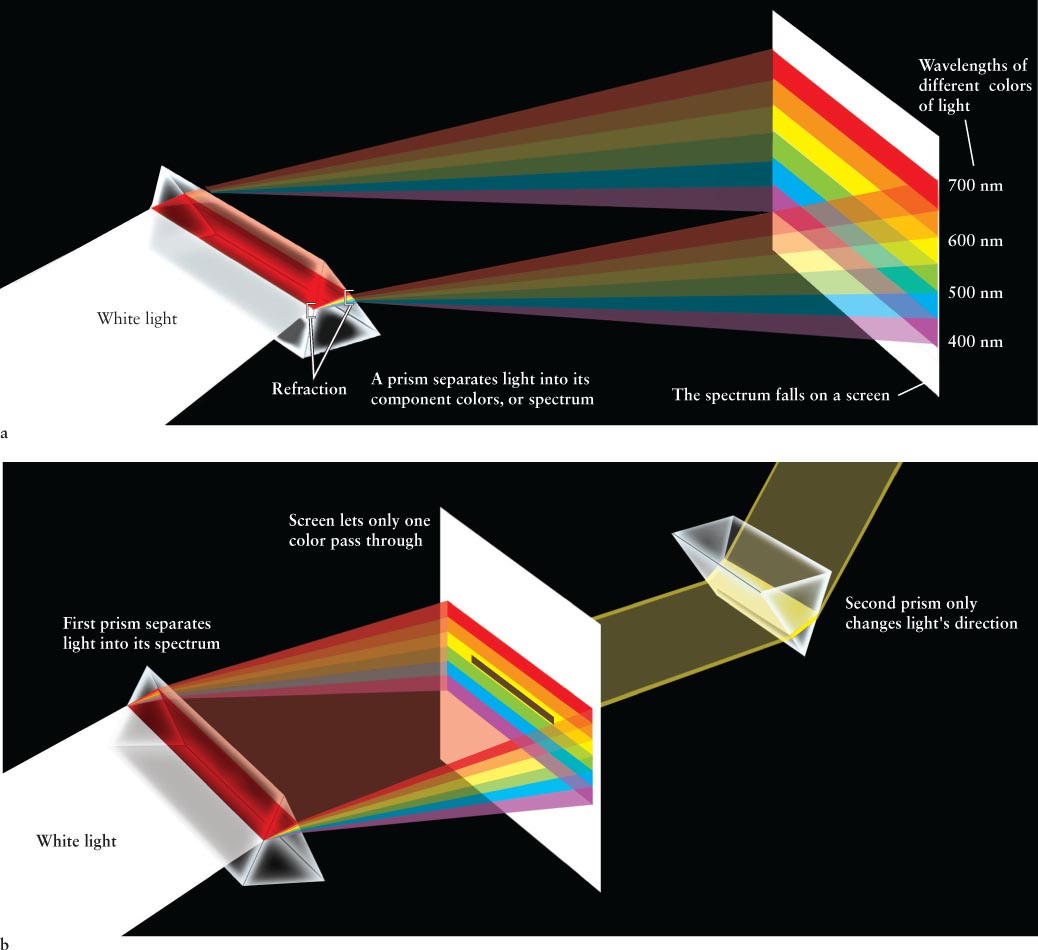
Figure 3-1 Prisms and a Spectrum (a) When a beam of white light passes through a glass prism, the light is separated or refracted into a rainbow-colored band called a spectrum. The numbers on the right side of the spectrum indicate wavelengths in nanometers (1 nm = 10–9 m). (b) This drawing of Newton’s experiment illustrates that glass does not add to the color of light, only changes its direction. Because color is not added, this experiment shows that color is an intrinsic property of light.
Then Newton selected a single color and sent it through a second prism (Figure 3-1b). The light that emerged from the first prism was refracted by the second prism, but it remained the same color. The fact that individual colors of refracted light were unchanged by the second prism led Newton to conclude that the colors of a full spectrum (shades of red, orange, yellow, green, blue, and violet; indigo is not considered a separate color in astronomy) were in fact properties of the light itself, and that white light is a mixture of colors. To prove this last point, he recombined all the spectrum colors, thereby recreating white light. Thus, different colors of the spectrum are different entities. But what, Newton wondered, is the nature of light that it could break apart into distinct colors and then be completely reconstituted?
Back in the mid-1600s, the Dutch scientist Christiaan Huygens proposed that light travels in the form of waves. Newton, on the other hand, performed many experiments in optics that convinced him that light is composed of tiny particles of energy. It turns out that both ideas were right.
In 1801, the English physicist Thomas Young demonstrated that light is indeed composed of waves. Young sent light of a single color through two parallel slits (Figure 3-2a). He reasoned that if the light were waves, then these waves would behave the way waves on the surface of water behave, flowing through similar gaps (Figure 3-2b). In particular, he theorized that the light waves from each slit would interact with light waves from the other. For example, when two light waves meet, with one wave going up and the other going down, they would interfere with each other and partially or totally cancel each other out, leaving dark regions on the screen. When two waves that are both moving up or both moving down meet, they would reinforce each other and create bright regions. As you can see in Figure 3-2, this is precisely what happened. (If light were just random particles going through the slits, they would not interfere with each other in this way, and the pattern on the screen would just yield two bright regions, one behind each slit.) The analogy with water waves ends here. For example, light waves do not have to travel through a medium, unlike water waves, which travel through the liquid medium of water.
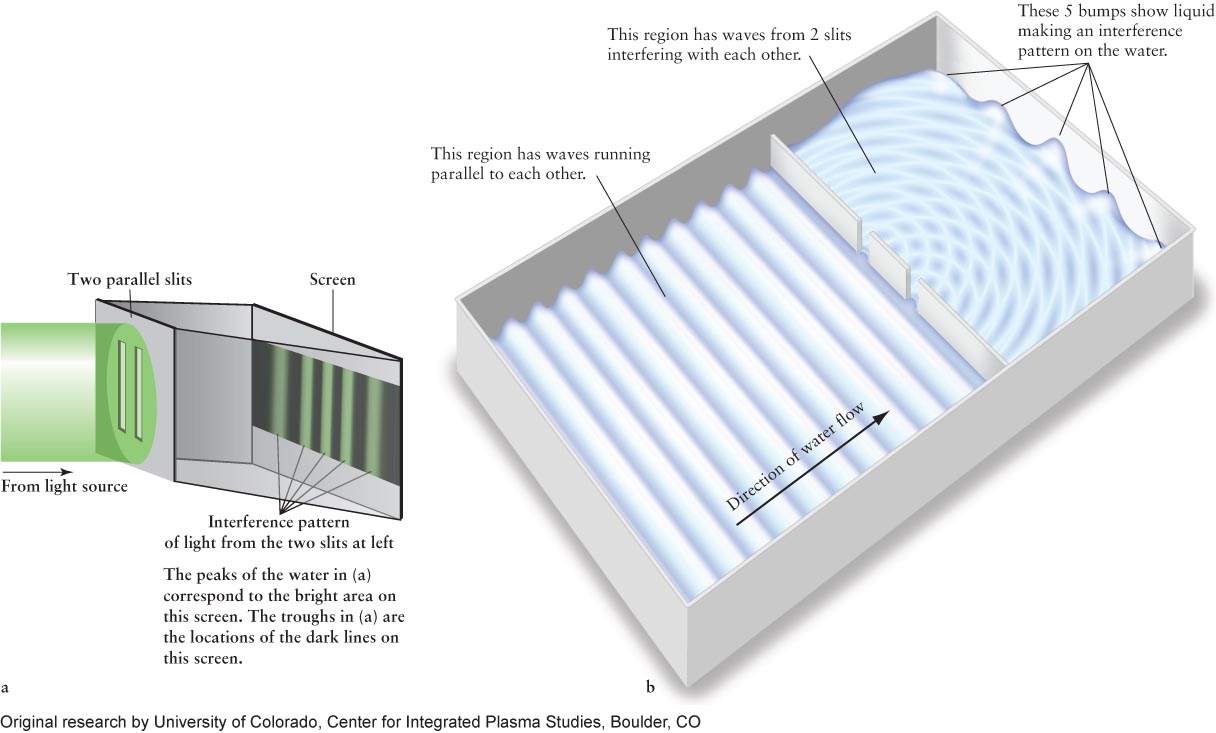
Figure 3-2 Wave Travel (a) Electromagnetic radiation travels as waves. Thomas Young’s interference experiment shows that light of a single color passing through a barrier with two slits behaves as waves that create alternating light and dark patterns on a screen. (b) Water waves passing through two slits in a ripple tank create interference patterns. The water waves interfere with each other, creating constructive interference (crests) and destructive interference (troughs) throughout the right side of the tank and on the far right wall.
Further insight into the wave character of light came from calculations by the Scottish physicist James Clerk Maxwell in the 1860s. Maxwell unified the descriptions of the basic properties of electricity and magnetism into four equations. By combining these equations, he demonstrated that electric and magnetic effects should travel through space together in the form of coupled waves (Figure 3-3) that have equal amplitudes. Maxwell’s suggestion that some of these waves, now called electromagnetic radiation, are observed as visible light was soon confirmed by a variety of experiments. Despite the name electromagnetic radiation, neither visible light nor any other type of electromagnetic radiation is electrically charged.
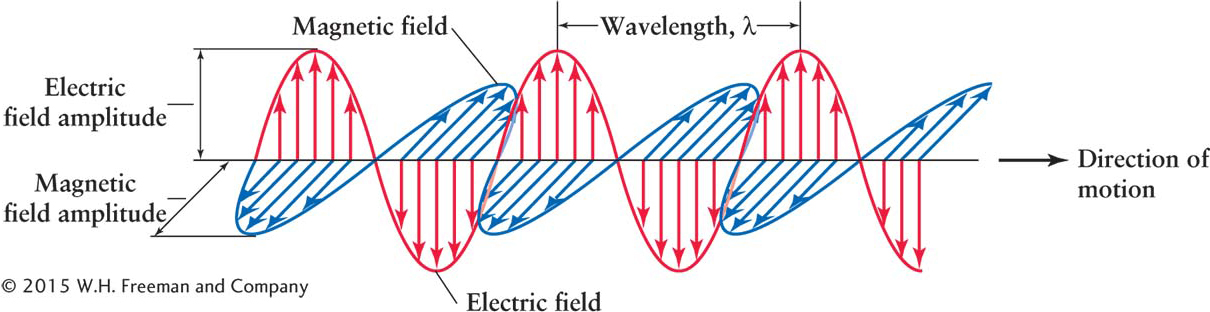
Figure 3-3 Electromagnetic Radiation All forms of electromagnetic radiation (radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays) consist of combined electric and magnetic fields oscillating perpendicular to each other and to the direction in which they move. In empty space this radiation travels at a speed of 3 × 105 km/s. These fields are the mathematical description of the electric and magnetic effects. The distance between two successive crests, denoted by λ, is called the wavelength of the light.
Newton showed that sunlight is composed of all the colors of the rainbow. Young, Maxwell, and others showed that light travels as waves. What makes the colors of the rainbow distinct from each other? The answer is surprisingly simple: Different colors are waves with different wavelengths. A wavelength, usually designated by λ, the lowercase Greek letter lambda, is the distance between two successive wave crests (see Figure 3-3).
The wavelengths of all colors are extremely small, less than a thousandth of a millimeter. To express these tiny distances conveniently, scientists use a unit of length called the nanometer (nm), where 1 nm = 10–9 m. Another unit you might encounter when talking to astronomers is the angstrom (Å), where 1 Å = 0.1 nm = 10–10 m. Experiments demonstrate that visible light has wavelengths ranging from about 400 nm for the shortest wavelength of violet light to about 700 nm for the longest wavelength of red light. Intermediate colors of the rainbow fall between these wavelengths (see Figure 3-1a). The complete spectrum of colors from the longest wavelength to the shortest is red, orange, yellow, green, blue, and violet. Referring to Figure 3-1, you can also see that the amount of refraction that different colors undergo depends on their wavelengths: The shorter the wavelength, the more the light is refracted.
3-2 Light travels at a finite but incredibly fast speed
The fact that we see lightning before we hear the accompanying thunderclap tells us that light travels faster than sound. But does that mean that light travels instantaneously from one place to another, or does it move with a measurable speed?
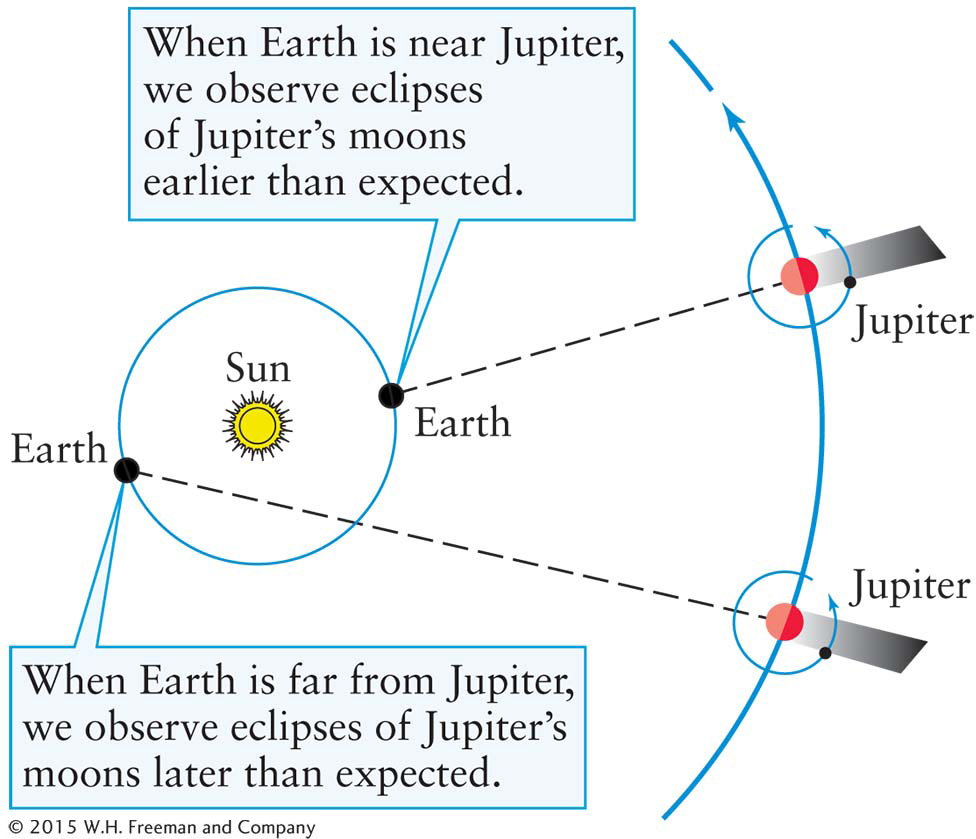
Figure 3-4 Evidence That Light Travels at a Finite Speed The times of the eclipses of Jupiter’s moons as seen from Earth depend on the relative positions of Jupiter, Earth, and the Sun. Rømer correctly attributed the variations in these times to the variations in the time that it takes light from these events to reach Earth.
Light speedThe first evidence for the finite speed of light came in 1675, when Ole Rømer, a Danish astronomer, carefully timed eclipses of Jupiter’s moons (Figure 3-4). Rømer discovered that the moment at which a moon enters Jupiter’s shadow depends on the distance between Earth and Jupiter. When Jupiter is in opposition—that is, when Jupiter and Earth are on the same side of the Sun (see Figure 2-4)—the Earth–Jupiter distance is relatively short compared to when Jupiter is near conjunction. At opposition, Rømer found that eclipses occur slightly earlier than predicted by Kepler’s laws, and they occur slightly later than predicted when Jupiter is near conjunction.
Focus Question 3-1
What color is refracted least?
Rømer correctly concluded that light travels at a finite speed, and so it takes more time to travel longer distances across space. The greater the distance to Jupiter, the longer the image of an eclipse takes to reach our eyes. From his timing measurements, Rømer concluded that it takes 162 minutes for visible light to traverse the diameter of Earth’s orbit (2 AU). Incidentally, Rømer’s interpretation of the data requires a heliocentric cosmology—that both Earth and Jupiter orbit the Sun.
Rømer’s subsequent calculation of the speed of light was off by 25% because the value for the astronomical unit (the average distance from Earth to the Sun) that existed at that time was highly inaccurate. Nevertheless, he proved his main point—light travels at a finite speed. The first accurate laboratory measurements of the speed of visible light were performed in the mid-1800s.
Maxwell’s equations also reveal that light of all wavelengths travels at the same speed in a vacuum (a region that contains no matter), and, despite a few atoms per cubic meter, the space between planets and stars is a very good vacuum. The constant speed of light in a vacuum, usually designated by the letter c, has been measured to be 299,792.458 km/s, which we generally round to
c = 3.0 × 105 km/s = 1.86 × 105 mi/s
(Standard abbreviations for units of speed, such as km/s for kilometers per second and mi/s for miles per second, will be used throughout the rest of this book.) Light that travels through air, water, glass, or any other substance always moves more slowly than it does in a vacuum.
The value c is a fundamental property of the universe. The speed of light appears in equations that describe, among other things, atoms, gravity, electricity, magnetism, distance, and time. Light has the extraordinary property that its speed is the same as seen by all observers. For example, if you were traveling in space away from the Earth at 99% of the speed of light, 0.99c, you would measure the speed of light moving toward you from all directions to be the same as the speed you would measure for that light if you were on Earth, namely, c. Likewise, you would measure the speed of light moving away from your spaceship in all directions to be the same as the speed of light moving away from you on Earth, both of which are also c.
Focus Question 3-2
Does a police siren approaching you sound higher in pitch (shorter wavelength) or lower in pitch than a siren at rest relative to you?
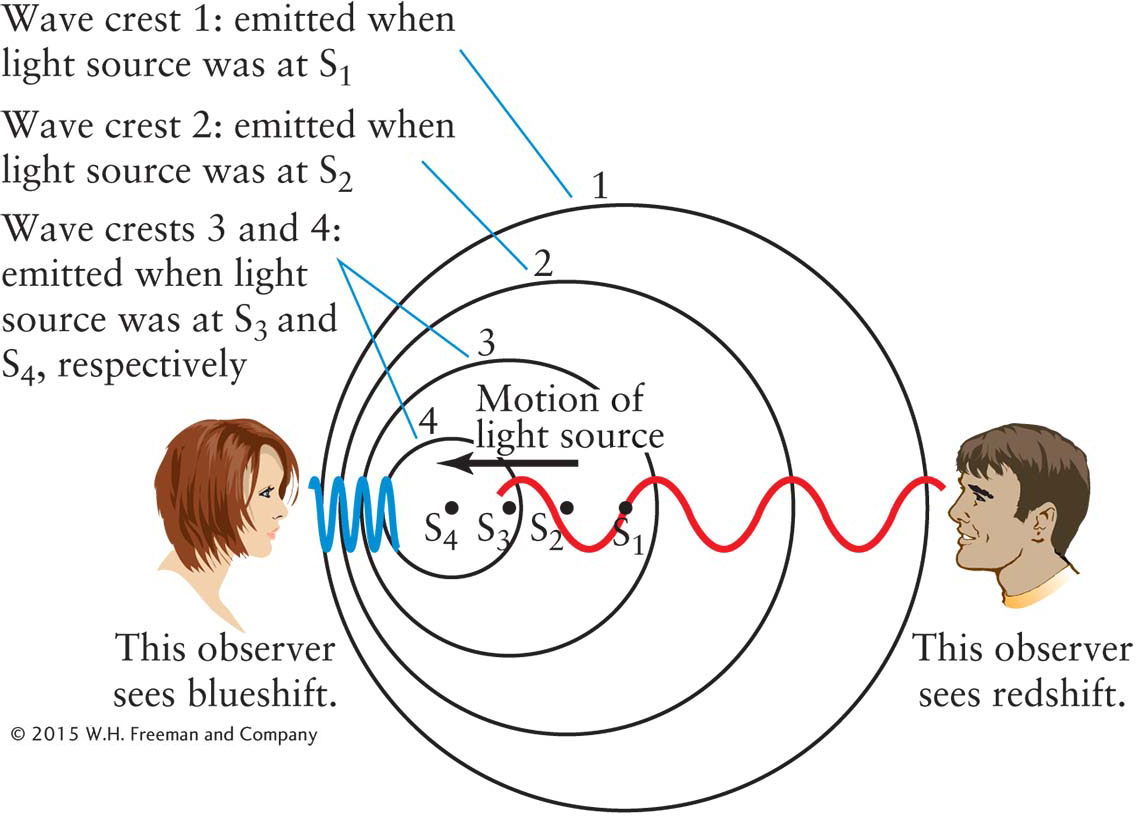
Figure 3-5 The Doppler Shift Wavelength is affected by motion between the light source and the observer. A source of light is moving toward the left. The four circles (numbered 1 through 4) indicate the location of the peaks of light waves that were emitted by the moving source when it was at points S1 through S4, respectively. Note that the waves are compressed in front of the source but stretched out behind it. Consequently, wavelengths appear shortened (blueshifted) if the source is moving toward the observer and lengthened (redshifted) if the source is moving away from the observer. Motion perpendicular to the observer’s line of sight does not affect wavelength.
The Doppler effectIn 1842, Christian Doppler, a professor of mathematics in Prague, deduced that wavelength, hence color, is affected by motion. As objects move toward or away from you, they change color, in analogy to how the pitch of a siren changes as it passes you. As shown for the observer on the left in Figure 3-5, the wavelengths of electromagnetic radiation from an approaching source are compressed. The circles represent consecutive wave peaks (S1 through S4) emitted in all directions as the source moves along. Because each successive wave is sent out from a position slightly closer to the female observer, she sees a shorter wavelength than she would if the source were stationary. All of the colors in the spectrum of an approaching source, such as a star, are therefore shifted toward the short-wavelength (blue) end of the spectrum, regardless of its distance. This phenomenon is called a blueshift.
Conversely, electromagnetic waves from a receding source are stretched out. The observer on the right in Figure 3-5 sees a longer wavelength than he would if the source were stationary. All of the colors in the spectrum of a receding source, regardless of its distance, are shifted toward the longer-wavelength (red) end of the spectrum, producing a redshift. A blueshift or a redshift is also called a Doppler shift. The amount of Doppler shift varies directly with approaching or receding speed: When the speeds are small compared to the speed of light, an object that approaches twice as fast as another has all of its colors (or wavelengths) blueshifted twice as much as does the slower-moving object. An object moving away twice as fast as another object has its colors redshifted twice as much as the slower-moving object. Doppler shift applies to spectral lines (discussed shortly) as well.
3-3 Einstein showed that light sometimes behaves as particles that carry energy
 By 1905, scientists were comfortable with the wave nature of light. However, in that year, Albert Einstein threw a monkey wrench into that theory when he proposed that light is composed of particles that have wave properties, creating what is now called the wave-particle duality. He used this idea to explain the photoelectric effect. Physicists knew that electrons are bound onto a metal’s surface by electric forces and that it takes energy to overcome those forces. Shorter wavelengths of light can knock some electrons off the surfaces of metals, while longer wavelengths of light cannot, no matter how intense the beam of long-wavelength light. Because some colors (or, equivalently, wavelengths) can remove the electrons and others cannot, the electrons must receive different amounts of energy from different colors of light. But how? Einstein proposed that light travels as waves enclosed in discrete packets, now called photons, and that photons with different wavelengths have different amounts of energy. Specifically, the shorter the wavelength, the higher a photon’s energy.
By 1905, scientists were comfortable with the wave nature of light. However, in that year, Albert Einstein threw a monkey wrench into that theory when he proposed that light is composed of particles that have wave properties, creating what is now called the wave-particle duality. He used this idea to explain the photoelectric effect. Physicists knew that electrons are bound onto a metal’s surface by electric forces and that it takes energy to overcome those forces. Shorter wavelengths of light can knock some electrons off the surfaces of metals, while longer wavelengths of light cannot, no matter how intense the beam of long-wavelength light. Because some colors (or, equivalently, wavelengths) can remove the electrons and others cannot, the electrons must receive different amounts of energy from different colors of light. But how? Einstein proposed that light travels as waves enclosed in discrete packets, now called photons, and that photons with different wavelengths have different amounts of energy. Specifically, the shorter the wavelength, the higher a photon’s energy.
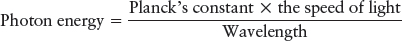
where Planck’s constant (named for the German physicist Max Planck) has the value 6.67 × 10–34 J · s, where J is the unit of energy called a joule, and the wavelength, the distance between wave crests or troughs, is shown in Figure 3-3. (J · s stands for joules multiplied by seconds, or joule-seconds.) Einstein’s concept of light, confirmed in numerous experiments, means that light can act both as waves (as when passing through slits) and as particles (as when striking matter).
The waves shown in Figure 3-3 are moving to the right. If you count the number of wave crests that pass a given point per second, you have found the frequency of the photon. The unit of frequency is the hertz, named in honor of the German physicist Heinrich Hertz. One hertz means that one cycle per second—or that one wave crest per second—passes any point. A thousand hertz means a thousand cycles per second, and so on. The frequency is used, among many other things, to identify radio stations. For example, WCPE radio in Wake Forest, North Carolina, has a frequency of 89.7 megahertz (a megahertz is a million hertz or a million cycles per second).
All photons with the same wavelength are identical to each other and, therefore, every photon of a given wavelength carries the same amount of energy as every other photon with that wavelength. The energy delivered by a photon is either enough to eject an electron from the surface of the metal or it is not; there is no middle ground. Extensive testing in the twentieth century confirmed both the wave and particle properties of light.
While the energy of a single photon is fixed by its wavelength, the total number of photons passing per second from that source with a given energy determines the intensity of the electromagnetic radiation at that wavelength (that is, how bright the object appears to be). The more photons detected, the higher the intensity, and vice versa. However, the intensity of light does not change the energy per photon. If one photon is unable to eject an electron from a metal, then billions of photons with that energy will still be unable to remove that electron.
3-4 Visible light is only one type of electromagnetic radiation

Figure 3-6  Experimental Evidence for Infrared Radiation This photograph shows the visible colors separated by a prism. The two thermometers in the region illuminated by visible light have temperatures less than the thermometer to the right of red. Therefore, there must be more radiation energizing (that is, heating) the warmest thermometer. This energy is what we call infrared radiation—invisible to the human eye, but detectable as heat.
Experimental Evidence for Infrared Radiation This photograph shows the visible colors separated by a prism. The two thermometers in the region illuminated by visible light have temperatures less than the thermometer to the right of red. Therefore, there must be more radiation energizing (that is, heating) the warmest thermometer. This energy is what we call infrared radiation—invisible to the human eye, but detectable as heat.
We now know that visible light occupies only a tiny fraction of the full range of possible wavelengths, collectively called the electromagnetic spectrum. Visible light has the range of wavelengths from 400 to 700 nm. However, Maxwell’s equations place no length restrictions on the wavelengths of electromagnetic radiation. What lies on either side of this interval? Around 1800, the British astronomer William Herschel discovered infrared radiation in an experiment with a prism. When he held a thermometer just beyond the red end of the visible spectrum, the thermometer registered a temperature increase, indicating that it was being heated by an invisible form of energy (Figure 3-6). Infrared radiation, discovered before Maxwell’s equations were formulated, was later identified as electromagnetic radiation with wavelengths slightly longer than red light. Our bodies detect infrared radiation as heat.
In experiments with electric sparks in 1888, Hertz succeeded in producing electromagnetic radiation a few centimeters in wavelength, now known as radio waves.
At wavelengths shorter than those of visible light, ultraviolet (UV) radiation extends from about 400 nm to 10 nm. In 1895, Wilhelm Roentgen invented a machine that produces electromagnetic radiation with wavelengths shorter than 10 nm, now called X-rays. Modern versions of Roentgen’s machine are found in medical and dental offices. X-rays have wavelengths between about 10 and 0.01 nm. Photons with even shorter wavelengths are called gamma rays. These boundaries are all arbitrary and primarily used as convenient divisions in the electromagnetic spectrum, which is actually continuous.
Focus Question 3-3
Which photon has more energy, an infrared photon or an ultraviolet photon?
As shown in Figure 3-7, the electromagnetic spectrum stretches from the longest-wavelength radio waves, through microwaves, infrared radiation, visible light, ultraviolet radiation, and X-rays, to the shortest-wavelength photons, gamma rays. On the long-wavelength side of the visible spectrum, infrared radiation covers the range from about 700 nm to 1 mm. Astronomers interested in infrared radiation often express wavelength in micrometers or microns (abbreviated μm), where 1 μm = 1000 nm = 10–6 m (one millionth of a meter). From roughly 1 mm to 10 cm is the range of microwaves. Microwaves are sometimes considered as a separate class of photons and sometimes categorized as infrared radiation or radio waves. Formally, radio waves are all electromagnetic waves longer than 10 cm.
The various types of electromagnetic radiation share many basic properties. For example, they are all photons, they all travel at the same speed, and they all sometimes behave as particles and sometimes as waves. But, because of their different wavelengths (and therefore different energies), they interact very differently with matter. For example, X-rays penetrate deeply into your body tissues, while visible light is mostly stopped and scattered by the surface layer of skin; your eyes respond to visible light but not to infrared radiation; and your radio detects radio waves but not ultraviolet radiation.
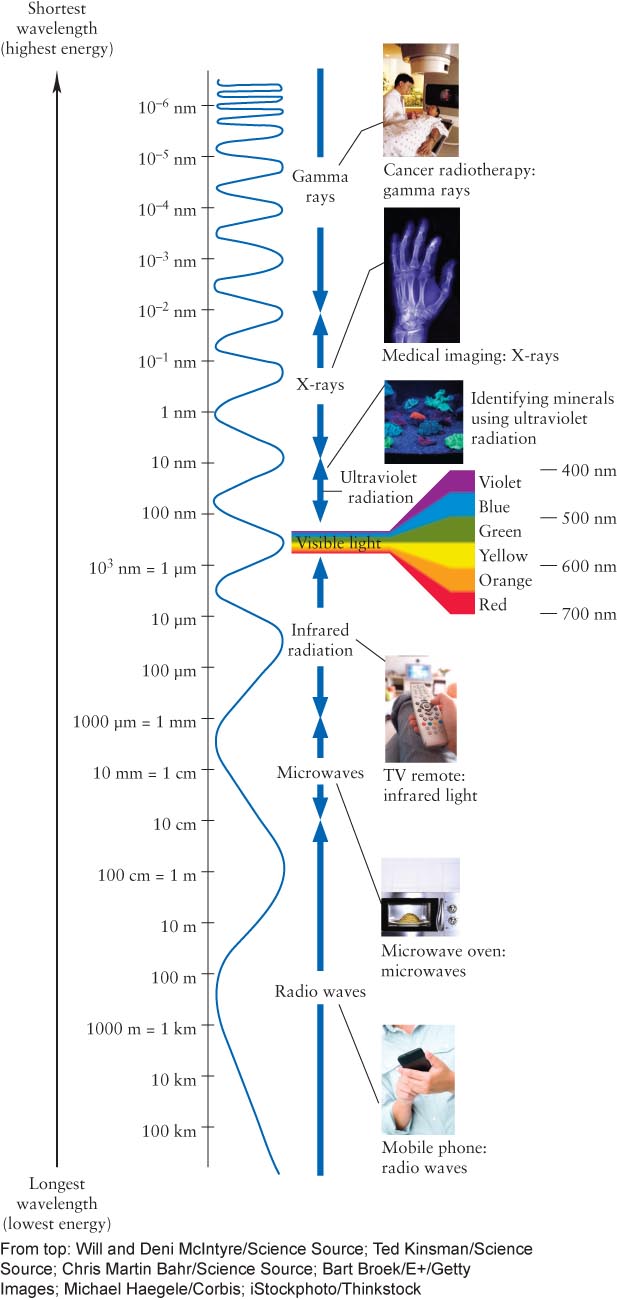
Figure 3-7 The Electromagnetic Spectrum The full array of all types of electromagnetic radiation is called the electromagnetic spectrum. It extends from the longest-wavelength radio waves to the shortest-wavelength gamma rays. Visible light forms only a tiny portion of the full electromagnetic spectrum. Note that 1 μm (micrometer) is 10-6 m, and 1 nm (nanometer) is 10-9 m. The insets show how we are now using all parts of the electromagnetic spectrum here on Earth.
Earth’s atmosphere is relatively transparent to visible light, radio waves, microwaves, short-wavelength infrared, and long-wavelength ultraviolet. As a result, these radiations pass through the atmosphere without much loss and can be detected by ground-based telescopes sensitive to them. Astronomers say that the atmosphere has windows for these parts of the electromagnetic spectrum (Figure 3-8).

Figure 3-8 “Windows” Through the Atmosphere Different types of electromagnetic radiation penetrate into Earth’s atmosphere in varying amounts. Visible light, radio waves, microwaves, short-wavelength infrared, and long-wavelength ultraviolet reach all the way to Earth’s surface. The other types of radiation are absorbed or scattered by the gases in the air at different characteristic altitudes (indicated by heights of windows). Although the atmosphere does not have actual windows, astronomers use the term to characterize the passage of radiation through it.
The longest-wavelength ultraviolet radiation, called UVA, causes tanning and sunburns. Ozone (O3) in Earth’s atmosphere normally screens out intermediate-wavelength ultraviolet radiation, or UVB. Until recently, the ozone in the ozone layer high in the atmosphere was being depleted by human-made chemicals, such as chlorofluorocarbons (CFCs) and bromine-rich gases. As a result, more UVB is reaching Earth’s surface, and these highly energetic photons severely damage living tissue, causing a surge in the rates of skin cancer and glaucoma, among other diseases.
 Earth’s atmosphere is completely opaque to the other types of electromagnetic radiation, meaning that they do not reach Earth’s surface. (This opacity is a good thing, because short-wavelength ultraviolet radiation [UVC], X-rays, and gamma rays are devastating to living tissue. Gamma rays, packing the highest energies per photon, are the deadliest.) Direct observations of these wavelengths must be performed high in the atmosphere or, ideally, from space.
Earth’s atmosphere is completely opaque to the other types of electromagnetic radiation, meaning that they do not reach Earth’s surface. (This opacity is a good thing, because short-wavelength ultraviolet radiation [UVC], X-rays, and gamma rays are devastating to living tissue. Gamma rays, packing the highest energies per photon, are the deadliest.) Direct observations of these wavelengths must be performed high in the atmosphere or, ideally, from space.
As noted earlier, photons with different energies interact with matter in different ways. Higher-energy photons will pass through or rip apart material from which lower-energy photons will bounce off. Telescope designs for collecting and focusing radiation, therefore, differ depending on the energies or, equivalently, the wavelengths of interest. Knowing the energies of photons and their effects on matter enables astronomers to design telescopes and recording devices sensitive to them. In the next section we will consider the lengths to which astronomers have gone to capture visible and invisible (or nonoptical) electromagnetic radiation.





 By 1905, scientists were comfortable with the wave nature of light. However, in that year, Albert Einstein threw a monkey wrench into that theory when he proposed that light is composed of particles that have wave properties, creating what is now called the wave-
By 1905, scientists were comfortable with the wave nature of light. However, in that year, Albert Einstein threw a monkey wrench into that theory when he proposed that light is composed of particles that have wave properties, creating what is now called the wave-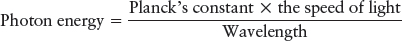



 Earth’s atmosphere is completely opaque to the other types of electromagnetic radiation, meaning that they do not reach Earth’s surface. (This opacity is a good thing, because short-
Earth’s atmosphere is completely opaque to the other types of electromagnetic radiation, meaning that they do not reach Earth’s surface. (This opacity is a good thing, because short- Experimental Evidence for Infrared Radiation This photograph shows the visible colors separated by a prism. The two thermometers in the region illuminated by visible light have temperatures less than the thermometer to the right of red. Therefore, there must be more radiation energizing (that is, heating) the warmest thermometer. This energy is what we call infrared radiatio
Experimental Evidence for Infrared Radiation This photograph shows the visible colors separated by a prism. The two thermometers in the region illuminated by visible light have temperatures less than the thermometer to the right of red. Therefore, there must be more radiation energizing (that is, heating) the warmest thermometer. This energy is what we call infrared radiatio