COMPARATIVE PLANETOLOGY
It often helps our understanding of complex topics, like planets, to see the big picture of how the major bodies are related to each other before exploring these objects in detail. To this end, we begin studying the eight planets in our solar system by comparing their physical properties. With this broad perspective in hand, we will then examine each planet and its accompanying moons individually. Earth and the three planets similar to it in chemistry, Mercury, Venus, and Mars, are studied in this chapter, whereas the larger planets, which are rock and metal (Earthlike) planets surrounded by substantial volumes of hydrogen, helium, and water, are explored in Chapter 7.
6-1 Comparisons of the eight planets show distinct similarities and significant differences
OrbitThe planets that newly forming the accretion of planetesimals in the young solar system were Mercury, Venus, Earth, Mars, Jupiter, Saturn, Uranus, and Neptune. As noted in Section 4-
|
|
Average distance from the Sun |
Sidereal Orbital period (Earth years) |
Orbital eccentricity (e) |
Orbital inclination |
||
|---|---|---|---|---|---|---|
|
|
(AU) |
(106 km) |
106 mi |
|||
|
Mercury |
0.39 |
58 |
36 |
0.24 |
0.206 |
7.01° |
|
Venus |
0.72 |
108 |
67 |
0.62 |
0.007 |
3.39° |
|
Earth |
1.00 |
150 |
93 |
1.00 |
0.017 |
0° |
|
Mars |
1.52 |
228 |
142 |
1.88 |
0.093 |
1.85° |
|
Jupiter |
5.20 |
778 |
483 |
11.86 |
0.048 |
1.30° |
|
Saturn |
9.54 |
1427 |
887 |
29.46 |
0.054 |
2.48° |
|
Uranus |
19.19 |
2871 |
1784 |
84.01 |
0.047 |
0.77° |
|
Neptune |
30.06 |
4497 |
2794 |
164.79 |
0.009 |
1.77° |
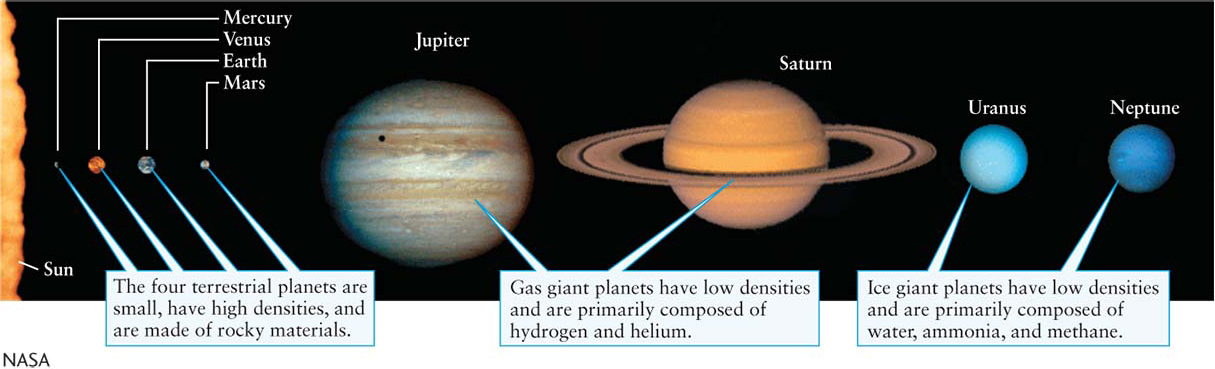
SizeThe planets fall into three size groups (Figure 6-1). The four inner planets form one group; Jupiter and Saturn, the gas giants, form the second group; and Uranus and Neptune, the ice giants, comprise the third, intermediate-
|
|
Diameter |
Mass |
Average density |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
(mi) |
(km) |
(Earth = 1) |
(lb)* |
(kg) |
(Earth = 1) |
(lb/yd3) |
(kg/m3) |
|
|
Mercury |
3031 |
4878 |
0.38 |
7.3 × 1023 |
3.3 × 1023 |
0.06 |
9150 |
5430 |
|
|
Venus |
7519 |
12,100 |
0.95 |
10.8 × 1024 |
4.9 × 1024 |
0.81 |
8850 |
5250 |
|
|
Earth |
7926 |
12,756 |
1.00 |
13.2 × 1024 |
6.0 × 1024 |
1.00 |
9300 |
5520 |
|
|
Mars |
4216 |
6786 |
0.53 |
14.1 × 1023 |
6.4 × 1023 |
0.11 |
6660 |
3950 |
|
|
Jupiter |
88,846 |
142,984 |
11.21 |
4.2 × 1027 |
1.9 × 1027 |
317.94 |
2240 |
1330 |
|
|
Saturn |
74,898 |
120,536 |
9.45 |
12.6 × 1026 |
5.7 × 1026 |
95.18 |
1160 |
690 |
|
|
Uranus |
31,763 |
51,118 |
4.01 |
19.2 × 1025 |
8.7 × 1025 |
14.53 |
2170 |
1290 |
|
|
Neptune |
30,775 |
49,528 |
3.88 |
2.2 × 1026 |
1.0 × 1026 |
17.14 |
2760 |
1640 |
|
* Pound (lb), as used here and most other places throughout this book, is a measure of mass, rather than weight.
MassA measure of the total amount of matter an object contains, its mass, is another characteristic that distinguishes the inner planets from the outer planets. The unit of mass in the metric system we use in this book is the kilogram. A slight complication occurs in describing mass in U.S. customary units. In those units the standard of mass is the slug, which is rarely used. Rather, we use the other unit of mass in U.S. customary units, the pound (lb, sometimes called the pound-
127
Focus Question 6-1
Venus and Mercury do not have any moons. What objects do you think astronomers observed being deflected in order to find the mass of these planets?
128
DensitySize and mass can be combined in a useful way to provide information about the chemical composition of a planet (or any other object). Matter composed of heavy elements, such as iron or lead, has more particles (protons and neutrons) and, hence, more mass packed into the same volume than does matter composed of light elements, such as hydrogen, helium, or carbon. Therefore, objects made primarily of heavier elements have a greater average density than objects composed primarily of lighter elements, where average density is given by the equation
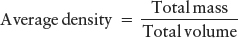
The chemical composition (kinds of elements) of any object determines its average density—how much mass the object has in a unit of volume. For example, a kilogram of rock takes up less space (is denser) than a kilogram of sugar, even though both have the same mass. Average density in metric units is expressed in kilograms per cubic meter (kg/m3). Common practice in the United States is to define density as pound/volume, such as lb/yd3, where pound here denotes mass, as discussed in the section on mass. For example, water has a density in metric units of 1000 kg/m3, which corresponds to roughly 1700 lb/yd3 in U.S. customary units. We will always present metric, and usually U.S. customary, numbers throughout the book.
To make the density of astronomical objects more comprehensible, it is often helpful to mentally compare these values with that of water, given above. The four inner planets have high average densities compared to water (see Table 6-
Focus Question 6-2
Why is Earth’s albedo continually changing?
In sharp contrast, Jupiter, Saturn, Uranus, and Neptune have relatively low average densities (see Table 6-
SpectraFurther information about the chemical composition of bodies in the solar system is obtained from their spectra. For solar system objects, spectra are provided primarily by sunlight scattered off their surfaces or the clouds that surround them. As we saw in Chapter 3, spectra provide us with details of an object’s surface (or atmospheric) chemical composition and rotation rate. From their spectra, we can confirm that the outer layers of the giant planets are primarily hydrogen and helium and that the surface of Mars is rich in iron oxides, among many other things.
AlbedoThe surfaces or the upper cloud layers of the various planets scatter (send in all directions) different amounts of light. The fraction of incoming light returning directly into space is called a body’s albedo. An object that scatters no light has an albedo of 0.0; for example, powdered charcoal has an albedo of nearly 0.0. An object that scatters all of the light that strikes it (a high-
MoonsEvery planet, except Mercury and Venus, has moons. At least 173 planetary moons are known to exist in the solar system (up from 99 known in 2001), and more are still being discovered. Table 6-3 lists the numbers of moons orbiting each planet. Unlike our Moon, most moons are irregularly shaped, and look more like potatoes than spheres. We will discover that there is as much variety among moons as there is among planets.
|
Mercury |
0 |
|
Venus |
0 |
|
Earth |
1 |
|
Mars |
2 |
|
Jupiter |
67 |
|
Saturn |
62 |
|
Uranus |
27 |
|
Neptune |
14 |
Insight Into Science
Astronomical Measurement Astronomers use the laws of physics to infer things we cannot measure directly. For example, we can get an overall idea of the chemical compositions of planets from their masses and volumes: Kepler’s laws enable us to determine the mass of a planet from the period of its moons’ orbits. The measured diameter of each planet (determined by its distance from Earth and its angular size in the sky) yields its volume. As shown in the equation earlier in this section, dividing total mass by total volume yields the average density. Comparing this density to the densities of known substances gives us information about the planets’ interior chemistries.
129