EARTH: A DYNAMIC, VITAL WORLD
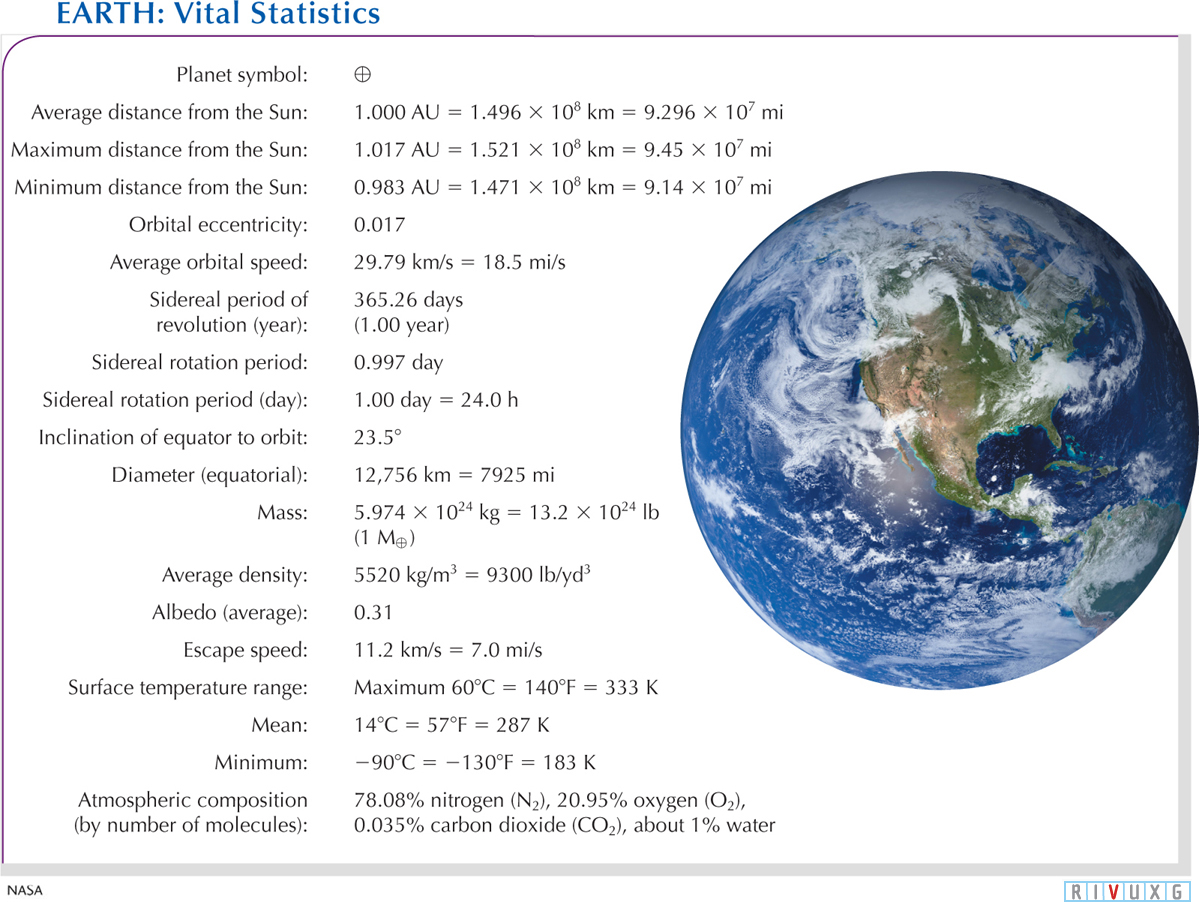
Figure 6-2 summarizes Earth’s important physical and orbital properties. The symbol ⊕ in Figure 6-
130
6-2 Earth’s atmosphere has evolved over billions of years
Earth’s atmosphere is a key part of our planet’s dynamic activity and its ability to sustain life. Essential to the existence of complex life is air that contains oxygen molecules. The air we breathe is predominantly a 4-
The theory of planet formation and the models that attempt to reproduce the early history of Earth indicate that the present atmosphere is the third one to envelop our planet. Earth’s earlier atmospheres contained no free oxygen. Composed of trace remnants of hydrogen and helium left over from the formation of the solar system, the first atmosphere did not last very long. The atoms and molecules in these gases were too light to stay near Earth. Heated by sunlight (meaning that they moved faster and faster), these particles gained enough kinetic energy to overcome Earth’s gravitational pull and escape into space.
The gases of the second atmosphere came primarily from inside Earth, vented through volcanoes and cracks in Earth’s surface. That atmosphere was composed primarily of carbon dioxide and water vapor, along with some nitrogen and other gases. All these gases consist of more massive atoms than the hydrogen and helium in the first atmosphere. Therefore, the speeds imparted to the gases of the second (and third) atmospheres by sunlight were not high enough to enable them to leave Earth’s gravitational bonds in large quantities. That is why Earth developed a permanent atmosphere.
There was roughly 100 times as much gas in that second atmosphere as there is in the air today. Carbon dioxide and water store a great deal of energy in the form of heat, helping to create what is called the greenhouse effect, about which we will say more shortly. The high concentration of these gases early in Earth’s history stored more heat than the atmosphere does today. Earth back then would have been a serious hothouse had the Sun reached its full intensity at that time. However, the Sun was dimmer and cooler in those days, so the high concentrations of carbon dioxide and water in the air actually prevented the young Earth from freezing.
Oceans began forming when water precipitated out of this atmosphere, as well as from impacts of water-
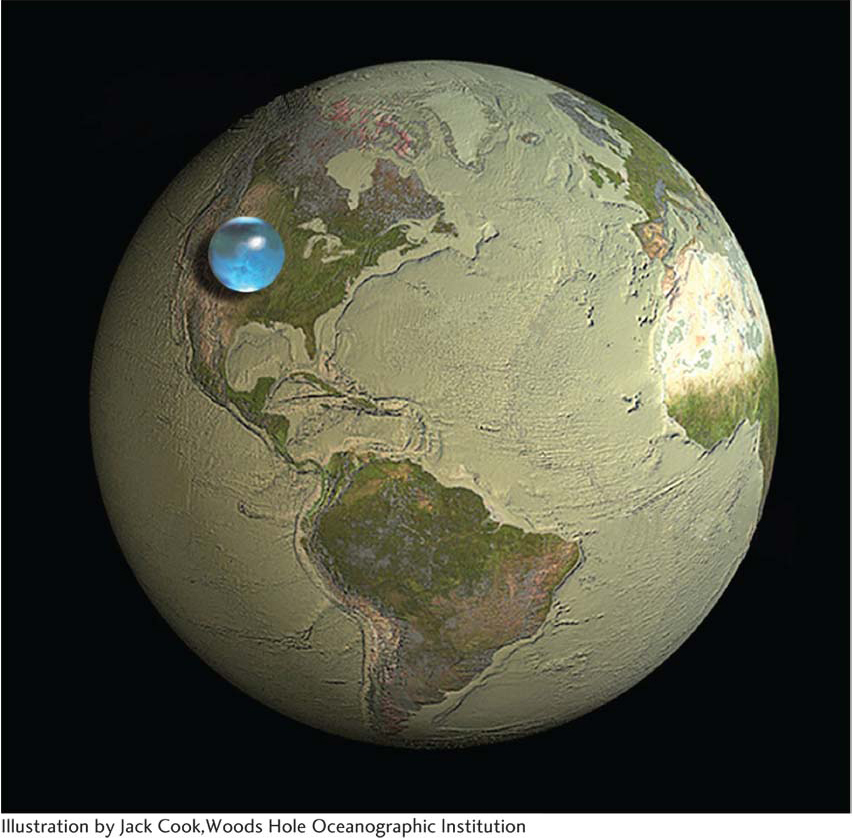
The oceans eventually soaked up about half the carbon dioxide in that second atmosphere, as the rain absorbed it from the air and carried it earthward. (Water holds a great deal of carbon dioxide, as you can see by the amount of carbon dioxide fizz in a can of soda.) Much of the carbon dioxide in the young oceans combined with water, silicon-
Early plant life in the oceans and then on the land removed most of the remaining carbon dioxide in the air, which plants used to create organic molecules that they needed in order to grow. The most efficient of such conversion mechanisms is photosynthesis, in which oxygen is released into the atmosphere as a waste product. The molecules that remained in the air as the carbon dioxide was being removed were mostly nitrogen, argon, and water vapor. Argon interacts very little with other atoms—
Oxygen that entered the air very early in Earth’s history came from oxygen-
131
About 2 billion years ago, after all of the surface minerals that could combine with oxygen had done so, the atmosphere began to fill with this gas. With the carbon dioxide reduced to a very low level, the air became the primarily nitrogen-
Because air has mass, it is pulled down toward Earth by gravity, thereby creating a pressure on the surface. Recall that we define pressure as a force acting over some area:
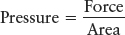
The weight of the air pushing down creates a pressure at sea level of 14.7 pounds per square inch, which is commonly denoted as 1 atmosphere (atm). That value is the pressure acting on your skin. The atmospheric pressure decreases with increasing altitude, falling by roughly half with every gain of 5.5 km (3.4 mi) of altitude. Seventy-
Lower layers of the atmosphereAll of Earth’s weather—
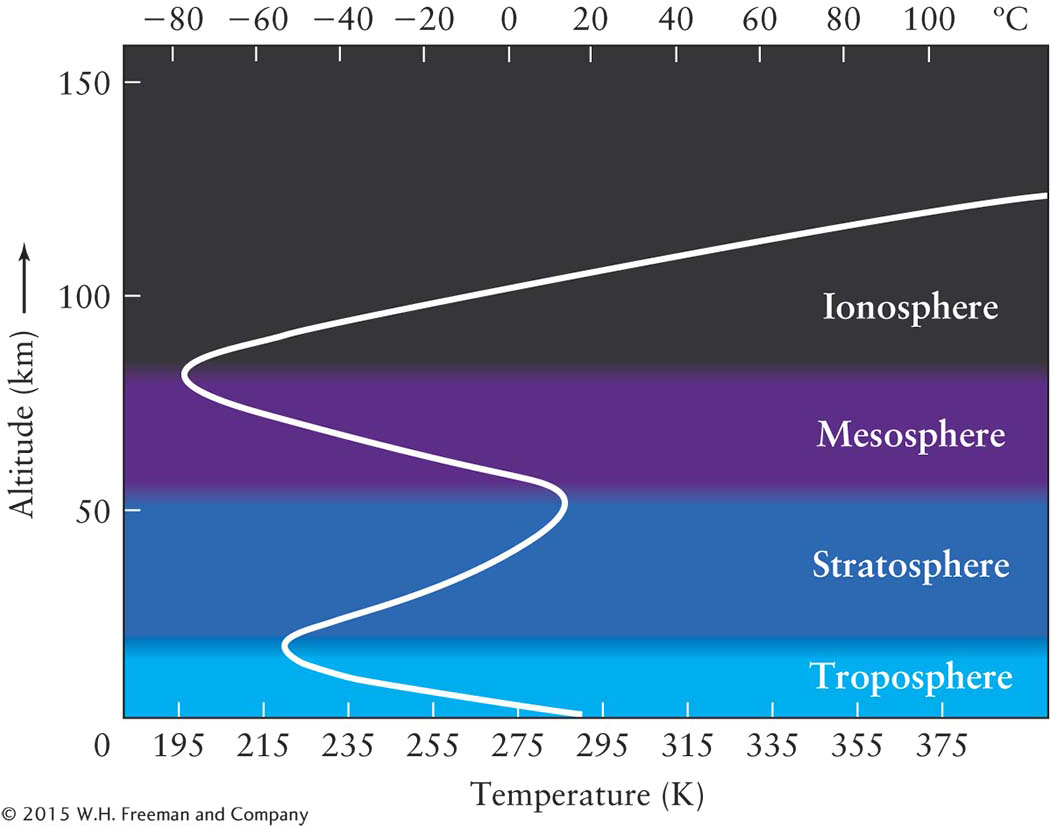
Starting at Earth’s surface, atmospheric temperature initially decreases as you go upward, reaching a minimum of 218 K (−67°F) at the top of the troposphere. Above this level is the region called the stratosphere, which extends from 11 to 50 km (approximately 7 to 31 mi) above Earth’s surface (Figure 6-4). This region is the realm of the ozone layer.
The ozone layerThe ozone layer has been much in the news in recent years. What is it, and how threatened is it? Ozone molecules (three oxygen atoms bound together, O3) are created in the stratosphere when short-
The troposphere (the lowest part of the atmosphere) is heated primarily by Earth, which is why the temperature in this part of the atmosphere decreases with altitude—
Not much ozone actually exists in the ozone layer. If all of the ozone there were compressed to the density of the air we breathe, it would be a layer only a few millimeters (about ⅛ inch) thick. This amount of ozone is enough, however, to protect us from most of the Sun’s lethal intermediate-
Insight Into Science
What’s in a Word? The word “layer” in the term ozone layer suggests a narrow region, perhaps a few meters thick. Not so. The ozone layer extends in altitude over some 40 km (25 mi) of the atmosphere!
Ultraviolet radiation has been a double-
Today, the stratospheric ozone layer is still vital, because the energy it absorbs would otherwise cause skin cancer and various eye diseases. In sufficiently high doses, ultraviolet radiation even damages the human immune system by destroying some of the molecules that normally repair cells in our bodies. Unfortunately, over the past few decades, the ozone layer has been partially depleted by human-
132
 Fortunately, as explained earlier, sunlight creates ozone. If current international efforts aimed at banning ozone-
Fortunately, as explained earlier, sunlight creates ozone. If current international efforts aimed at banning ozone-
There is actually a second layer of ozone that has been developing over the past few centuries, which is hazardous to our health. It is being generated by vehicle and industrial emissions. This ozone is located in the troposphere, along with other air pollution. Unlike the ozone in the stratosphere, the tropospheric ozone is damaging plants and causing respiration problems for animals, including humans. This ozone also contributes to global warming.
Upper layers of the atmosphereNote in Figure 6-
Carbon dioxide and the greenhouse effect todayPerhaps you have had the experience of parking your car in the sunshine on a warm summer day. You roll up the windows, lock the doors, and go on an errand. After a few hours, you return to discover that the interior of your automobile has become considerably hotter than the outside air temperature (Figure 6-5).
What happened to make your car so warm? First, sunlight entered it through the windows. This radiation was absorbed by the dashboard and the upholstery, increasing their temperatures. Because they become warm, your dashboard and upholstery emit infrared radiation, which you detect as heat and which your car windows do not permit to escape. This energy is therefore trapped inside your car and absorbed by the air and interior surfaces. As more sunlight comes through the windows and is trapped, the temperature continues to increase. The same trapping of sunlight warms greenhouses.
The greenhouse effect in our atmosphere works like this. All wavelengths of electromagnetic radiation from the Sun approach Earth’s atmosphere. Some of this energy is scattered by clouds, land, and water right back into space (Figure 6-6a), while some is absorbed in the atmosphere, thereby heating it. Some visible light, along with some ultraviolet radiation and radio waves, penetrates to Earth’s surface and is absorbed by it. This energy helps heat the planet (along with heat from the air and from inside the planet).
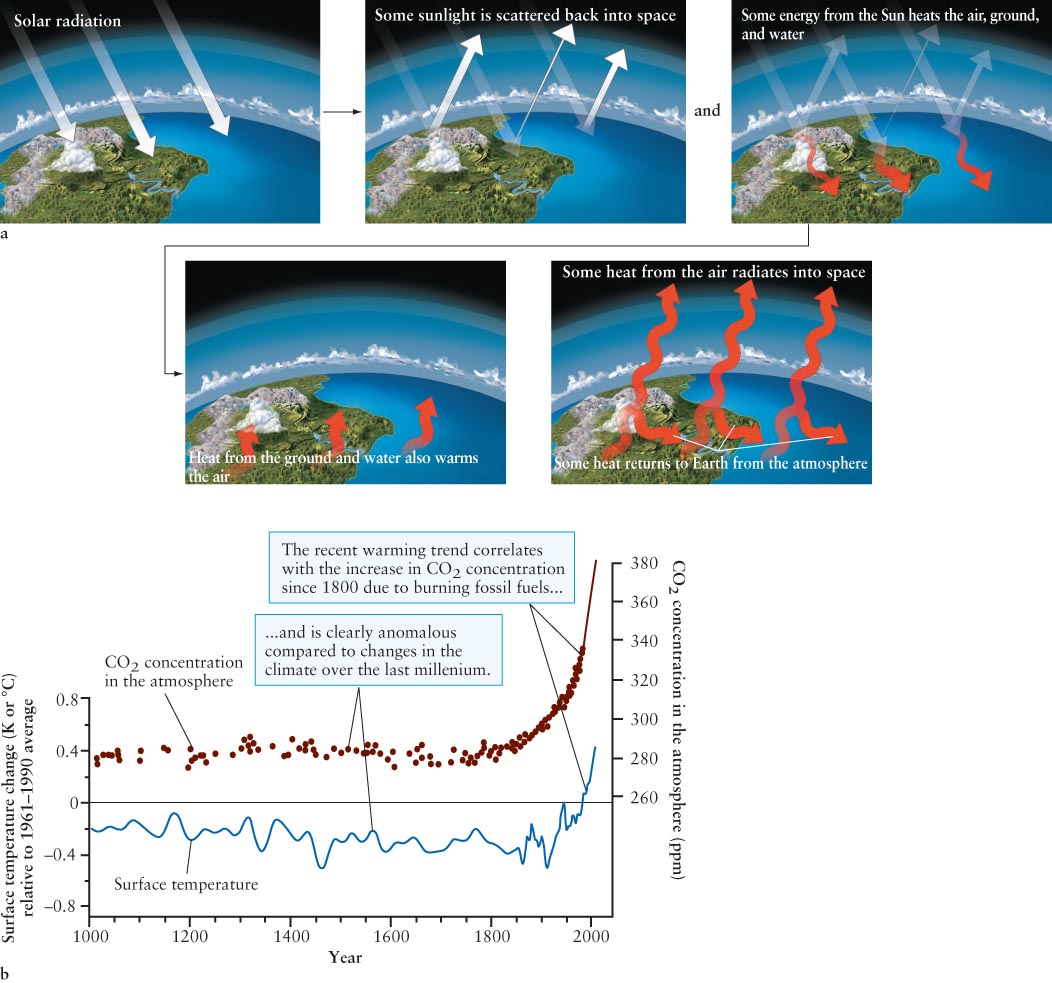
Behaving nearly like a blackbody, Earth radiates energy primarily as infrared radiation (where its blackbody spectrum peaks). Unlike most visible light, which passes relatively easily through the gases in our atmosphere, infrared radiation is absorbed by certain gases, especially water, carbon dioxide, and ozone in the air. The energy from these photons further heats the atmosphere. Carbon dioxide levels are increasing in Earth’s atmosphere, primarily as a result of human activity, such as the burning of fossil fuels. Increased atmospheric carbon dioxide stores more heat in the air, and the air becomes warmer. Normally, carbon dioxide is removed from the air (and oxygen added) by the process of photosynthesis in green leaves. With increased deforestation and the expansion of cities, fewer plants and trees are left, so the carbon dioxide in the air remains there longer, overheating the air and creating global warming. Figure 6-
The consequences of the recent global warming trend are quite complex and serious. They include the melting of glaciers and polar ice caps, rising sea levels, changes in ocean heating and circulation patterns, changes in weather patterns worldwide, and increases in undesirable weather events, such as hurricanes, tornadoes, droughts, floods, heat waves, and shoreline erosion. With scientific data on the human effects that add to global warming in hand, many nations of the world are working together to decrease its causes.
133
6-3 Plate tectonics produce major changes on Earth’s surface
Focus Question 6-3
What effect will an increase in the amount of vegetation on Earth have on the carbon dioxide level in the atmosphere?
Large landmasses called continents protrude through Earth’s oceans. These landmasses are regions of Earth’s outermost layer, or crust, that are composed of relatively low-
The crust has distinct boundaries (an example is shown in Figure 6-7), many occurring beneath Earth’s oceans. These divisions suggest that the continents are separate bodies. Earthquakes and volcanoes often occur near the boundaries, hinting at activity taking place below Earth’s crust.
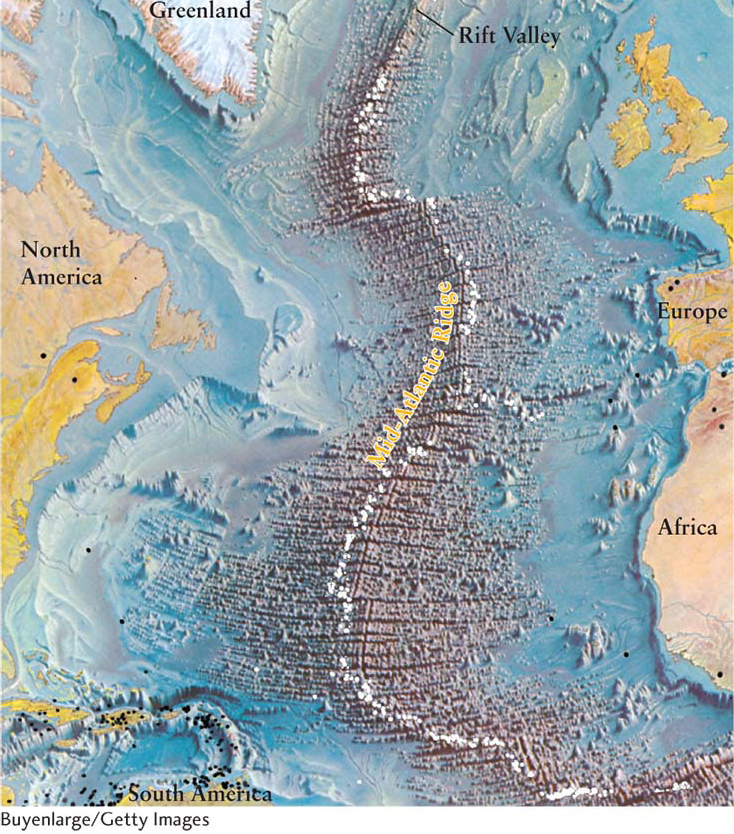
Anyone who carefully examines the shapes of Earth’s continents might conclude that landmasses move. Eastern South America, for example, looks like it would fit snugly against western Africa. Between 1912 and 1915, the German scientist Alfred Wegener published his observations on this remarkable fit between landmasses on either side of the Atlantic Ocean, an idea also proposed by Isaac Newton two centuries earlier. Wegener was inspired to advocate the hypothesis of continental drift—the idea that the continents on either side of the Atlantic Ocean have moved apart.
134
Wegener argued that a single, gigantic supercontinent called Pangaea (pronounced pan-
Then, in the mid-
Insight Into Science
Acceptance of Scientific Theories Before they are accepted, scientific theories may undergo years or decades of testing and replication. Newton speculated about continental drift in the late seventeenth century. Wegener had proposed continental drift by 1912. His hypothesis was not verified until the 1950s and was not widely accepted until the late 1960s.
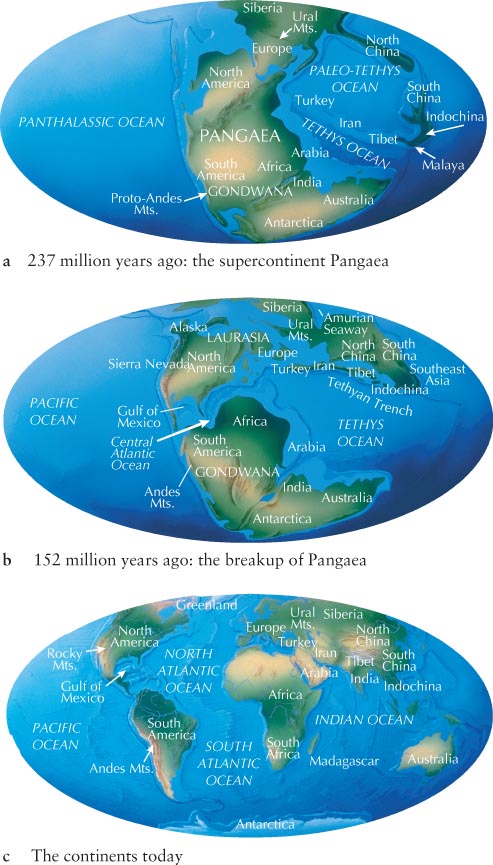
Seafloor spreading provided the physical mechanism that allowed for Wegener’s hypothesis of crustal motion to be developed into the modern theory of plate tectonics. It was after this hypothesis was confirmed in the 1960s that the theory of continental drift became widely accepted.
Earth’s surface is made up of about a dozen major thick rafts of rock called tectonic plates that move relative to one another. These plates float on the slowly moving, denser matter below them. The plates are not always separate, as they are today. Sometimes they merge into a single mass called a supercontinent. In recent years, geologists have uncovered evidence that points to a whole succession of supercontinents that broke apart and reassembled. Pangaea is only the most recent supercontinent in this cycle, which repeats on average every 500 million years (Figure 6-8).
The boundaries between tectonic plates are the sites of some of the most impressive geological activity on our planet. Earthquakes and volcanoes tend to occur at these boundaries, where the plates are colliding (at convergent boundaries), separating (at divergent boundaries), or grinding against each other (at transform-
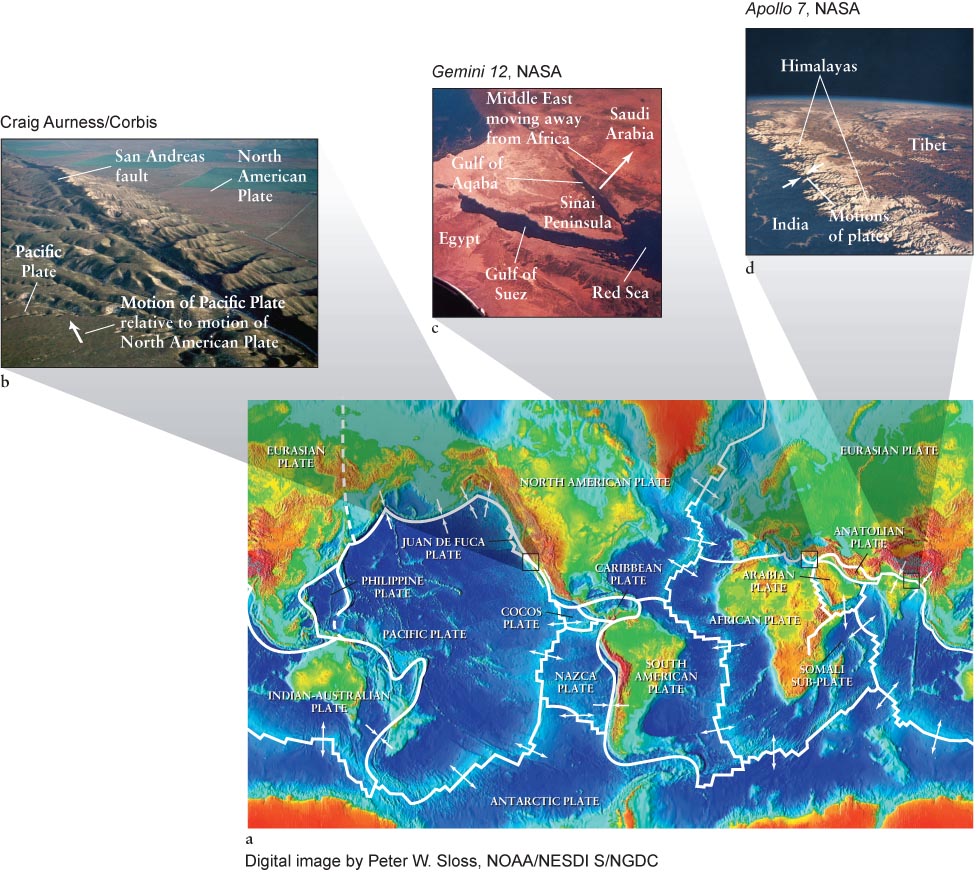
135
Countless impacts have occurred in the oceans, which cover most of our planet. Some of the impacting bodies were slowed or obliterated before cratering the ocean floor, but others were large enough or fast enough to hit the floor. Tectonic plate motion helps explain why Earth’s oceans have so few craters today. The resulting craters in the oceans rode on their tectonic plates until they reached a convergent boundary, where they were subducted (drawn underground) and thereby erased.
Impact craters created on the dry landmasses (continents and islands) are exposed to the atmosphere. Wind and water erode them, with silt from rivers helping to cover any remnants. Only the youngest craters on Earth remain visible.
What powers all of this activity? Some enormous energy source must be shifting landmasses, and it must have been doing so for billions of years. The answer to that question leads us to a scientific model of Earth’s interior.
6-4 Earth’s interior consists of a rocky mantle and an iron-rich core
Geologists have calculated that Earth was composed entirely of liquid rock and metal soon after its formation, about 4.6 billion years ago. The violent impact of space debris, the compression of the interior by the mass above it, and the energy released by the breakup of radioactive elements due to radioactive decay and nuclear fission heated, melted, and kept the young Earth molten. In radioactive decay, radioactive nuclei emit energy and small particles, such as electrons, protons, neutrons, or helium nuclei. In nuclear fission, the atomic nuclei break into substantial pieces, such as when a uranium atom breaks into a rubidium atom and a cesium atom, while also releasing other particles and energy. Fission also creates the heat used to generate electricity in nuclear power plants and some spacecraft, like the Mars rover Curiosity. That process differs from how energy is generated by nuclear fusion in the Sun, wherein lighter atoms are fused together, as mentioned in Chapter 4 and discussed in detail in Chapter 9.
Most of the iron and other dense elements sank toward the center of the young, liquid Earth, just as a rock sinks in a pond. At the same time, most of the less dense materials were forced upward toward the surface (Figure 6-10a). This process, called planetary differentiation, produced a layered structure within Earth: a very dense central core surrounded by a mantle of less dense minerals, which, in turn, was surrounded by a thin crust of relatively light minerals (Figure 6-
Focus Question 6-4
What type of boundary exists between Africa and the Middle East?
Differentiation explains why most of the rocks you find on the ground are composed of lower-
136
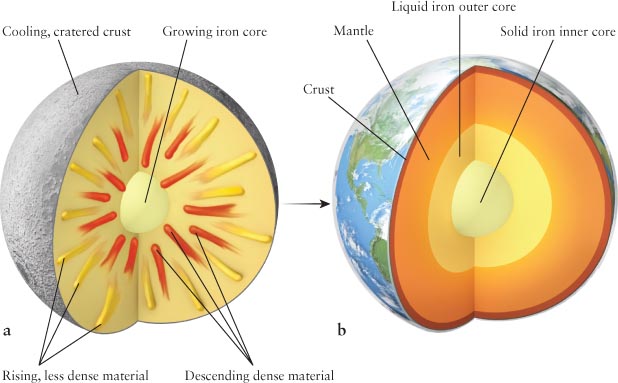
To understand Earth’s interior, geologists have created a mathematical model that includes the effects of differentiation, gravity, radioactivity, chemistry, heat transfer, rotation, and other factors. The model predicts that Earth’s temperature increases steadily from about 290 K (62°F) on the surface to nearly 6000 K (10,800°F) at the center. Compression of the core, as noted earlier, provides much of the heat that remains there, while radioactivity replenishes most of the heat lost from the mantle through the planet’s surface. In addition to temperature, pressure also increases with increasing depth below Earth’s surface because the deeper you go, the more mass presses down on you. These factors shape Earth’s inner layers.
You might think that a temperature of 6000 K (10,800°F) would melt virtually any substance. The mantle, in particular, is composed largely of minerals rich in iron and magnesium, both of which have melting points of only slightly more than 1250 K (1790°F) at Earth’s surface. However, the melting point of any substance also depends on the pressure to which it is subjected: the higher the pressure, the higher a material’s melting point. The high pressures within Earth (1.4 million atm at the bottom of the mantle) keep the mantle relatively solid to a depth of about 2900 km (1800 mi). This region still deforms, but more slowly than the rock directly below it, which has the consistency of Silly Putty. The mantle rests on a liquid iron outer core, below which the pressure is so high that it compresses the very hot iron atoms there to form the solid inner core (Figure 6-
137
Geologists have tested their models of Earth’s interior by studying the response of the planet to earthquakes. These events produce a variety of seismic waves, vibrations that travel through Earth either as ripples, like ocean waves, or by compressing matter, like sound waves. Geologists use sensitive seismographs to detect and record these vibratory motions. The varying density and composition of Earth’s interior affect the direction and speed of seismic waves traveling through Earth. By studying the deflection of these waves, geologists have been able to determine the structure of the planet’s interior.
Analysis of seismic recordings led to the discovery that Earth’s solid core has a radius of about 1250 km (775 mi), surrounded by a molten iron core with a thickness of about 2250 km (1375 mi). For comparison, the overall radius of our planet is 6378 km (3963 mi). The cores are composed of roughly 80% iron and 20% lighter elements, such as nickel, oxygen, and sulfur. The interior of our planet therefore has a curious structure: a liquid region sandwiched between a solid inner core and a solid mantle (see Figure 6-
A sophisticated computer model of Earth’s interior predicts that the solid inner core is rotating faster than the rest of the planet. Seismic evidence supports the model that the core spins around about 2° more per year than the surface. Such observations as well as refined models are revealing more of the secrets of Earth’s interior.
The tremendous heat generated inside Earth provides the energy that drives the motion of the tectonic plates in Earth’s crust. This heat is transported upward by convection. In this process a liquid or gas is heated from below and, as a result, it expands, becomes buoyant, and therefore rises, carrying the heat it received with it. Eventually, the liquid or gas releases the heat, cools, condenses, and sinks back down (subducts). This motion of fluid causes a circular current, called a convection current, to arise. You witness convection every time you see liquid simmering in a pot. Heated from below, blobs of hot fluid or gas move upward. The rising material releases its heat at the surface (Figure 6-11a).
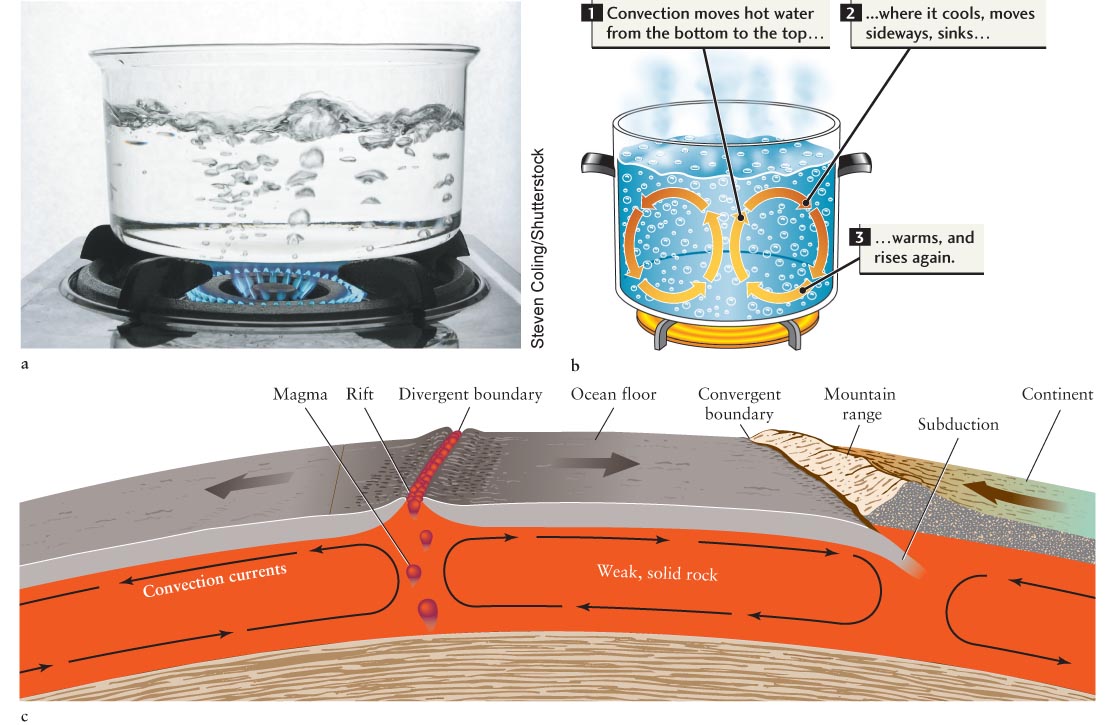
Earth’s upper mantle, upon which the tectonic plates lie, is solid rock. However, this rock is weak and hot enough to have an oozing, plastic flow (like stretching Silly Putty). The crust and this upper mantle are collectively called the lithosphere. As sketched in Figure 6-
Supercontinents like Pangaea apparently sow the seeds of their own destruction by blocking the flow of heat from Earth’s interior. As soon as a supercontinent forms, temperatures beneath it increase. As heat accumulates, the supercontinent domes upward and cracks. Overheated molten rock wells up to fill the resulting rifts, which continue to widen as pieces of the fragmenting supercontinent are pushed apart.
Earth’s molten, iron-
6-5 Earth’s magnetic field shields us from the solar wind
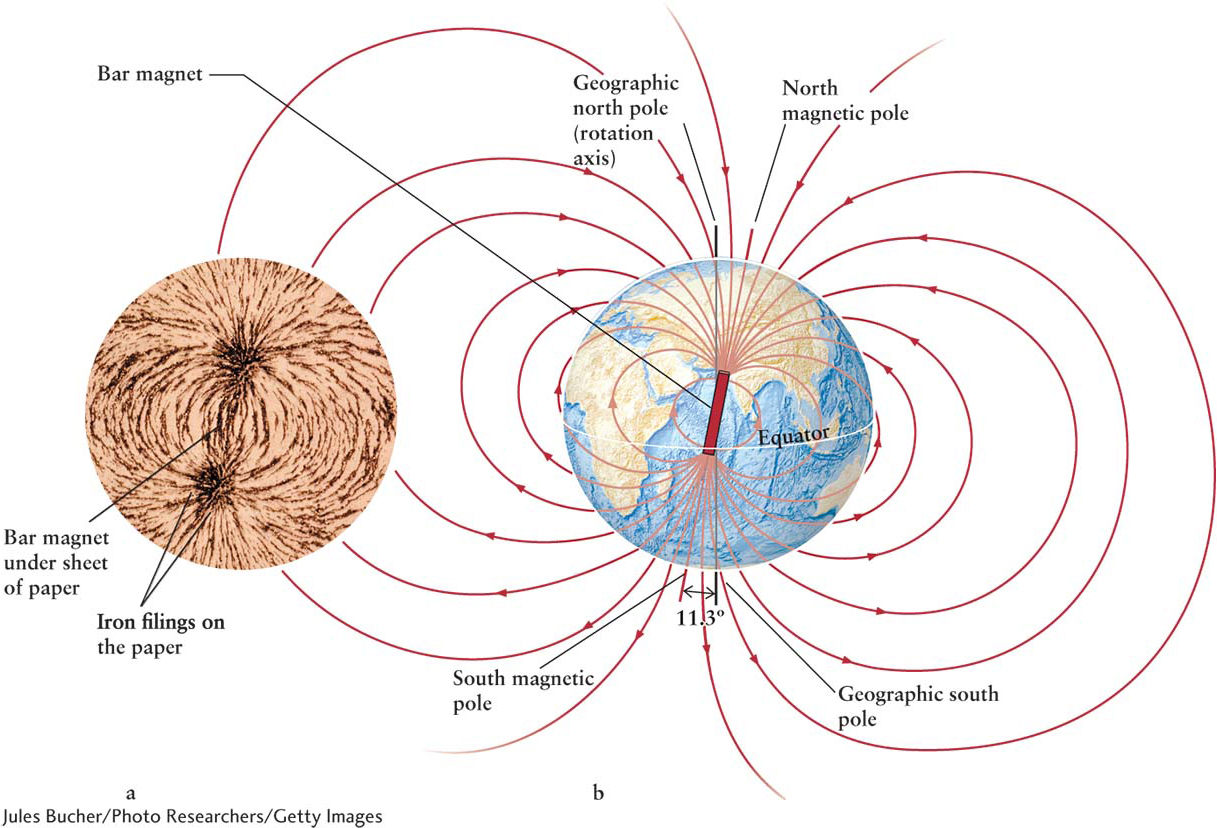
If you have ever used a compass, you have seen the effect of Earth’s magnetic field. This planetary field is quite similar to the field that surrounds a bar magnet (Figure 6-12a). Earth’s magnetic field is created by electric charges in motion inside the planet. Analogously, the electric wires in your house also carry moving charges called electric currents that, in turn, create magnetic fields of their own.
138
Many geologists believe that convection of molten iron in Earth’s outer core, combined with our planet’s rotation, creates the electric currents, which, in turn, create Earth’s magnetic field. The details of this so-
The magnetic fields near Earth’s south rotation pole loop out tens of thousands of kilometers into space and then return near Earth’s north rotation pole. The places where the magnetic fields pierce Earth’s surface most intensely are called the north and south magnetic poles. These poles move continuously relative to our planet’s rotation poles. Evidence in solidified lava reveals that Earth’s magnetic field completely reverses or flips (the north magnetic pole becomes the south magnetic pole and vice versa) on an irregular schedule, typically occurring every 200,000 to 300,000 years. The last reversal is an exception, having taken place about 780,000 years ago. Each flip takes about 4000 years to complete. Today, the magnetic and rotation poles of Earth are about 11.3° apart; some planets have much larger angles between their magnetic and rotation poles. Figure 6-13 is a scale drawing of the magnetic fields around Earth, which comprise Earth’s magnetosphere. Magnetic fields always form complete loops. (The unconnected lines in Figure 6-
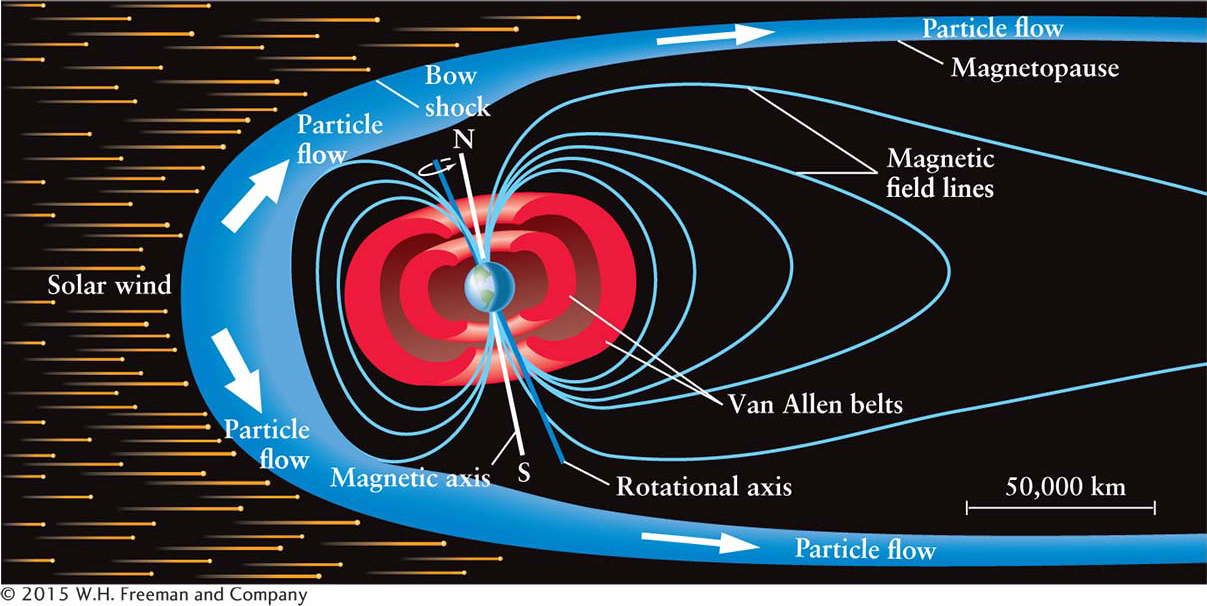
Our planet’s magnetic field protects Earth’s surface from bombardment by particles flowing from the Sun (see Chapter 9). The solar wind is the erratic stream of electrically charged particles ejected from the Sun’s upper atmosphere. Near Earth, the particles in the solar wind move at speeds of approximately 400 km/s, or about a million miles per hour.
139
Magnetic fields can change the direction of moving charged particles. Thus, solar wind particles heading toward Earth are deflected by our planet’s magnetic field. Many particles, therefore, pass around Earth (see Figure 6-
140
Earth’s magnetic field also traps some of the solar wind particles in two huge, doughnut-
Focus Question 6-5
What happens to the Van Allen belts when Earth’s magnetic field is flipping?
When the flow of particles from the Sun is particularly high, some of the solar wind that is trapped in the Van Allen belts is funneled toward Earth’s polar regions where these particles descend and cascade down into Earth’s upper atmosphere, usually in a ring-

Insight Into Science
Pace of Discovery Discoveries such as the Van Allen belts remind us how much remains to be learned, even about our own planet. Such fundamental discoveries continue to be made for many reasons. For example, the technology necessary to make certain observations may just have been developed, or our equations that describe various phenomena may become more complete. Sometimes these equations are so complex that their implications are not immediately clear. Progress in science requires unquenchable curiosity and unfailing open-
Occasionally, violent events on the Sun’s surface, called solar flares and coronal mass ejections, send bursts of protons and electrons straight through the Van Allen belts and into the atmosphere. These solar events partially deplete the ozone layer and cause spectacular auroral displays that can often be seen over a wide range of latitudes and longitudes. These solar events also disturb radio, TV, and cell phone transmissions and can damage communications satellites and electric transmission lines on Earth.
In this era of burgeoning satellite usage and high technology here on Earth, the effects of the Sun’s electromagnetic radiation, along with the flow of particles from it, are increasingly important. As a result, space weather satellites are now continually monitoring our neighborhood in space and warning about solar events that could imperil humans and machines in space and equipment, like power grids, here on Earth.
141
 Earth’s Vital Statistics The planet symbol for Earth is ⊕. Other planets have different symbols; these are all used as shorthand for information about each world. For example, the mass of Earth is denoted M⊕. This figure provides information about Earth’s physical and orbital properties. Recall that pounds (lb) here are a unit of mass. Astronauts spend many hours watching our world as they orbit it. The image here gives you an idea of why they find it so fascinating.
Earth’s Vital Statistics The planet symbol for Earth is ⊕. Other planets have different symbols; these are all used as shorthand for information about each world. For example, the mass of Earth is denoted M⊕. This figure provides information about Earth’s physical and orbital properties. Recall that pounds (lb) here are a unit of mass. Astronauts spend many hours watching our world as they orbit it. The image here gives you an idea of why they find it so fascinating.