THE SUN’S INTERIOR
During the 1800s, geologists and biologists found convincing evidence that Earth must have existed in more or less its present form for at least hundreds of millions of years. This fact posed severe problems for astrophysicists, because at that time it seemed impossible to explain how the Sun could continue to shine for so long, radiating immense amounts of energy into space as it does. If the Sun were shining by burning coal or hydrogen gas, it would be ablaze for only 5000 years before consuming all of its fuel.
Everyday experience tells us that the Sun is the source of an enormous amount of energy. We have just seen that observations reveal hot gas, intense magnetic fields, and a variety of continuous and transient features on the Sun’s surface. But the energy does not come from the surface gas or the magnetic fields—
9-7 Thermonuclear reactions in the core of the Sun produce its energy
In 1905, Albert Einstein provided an important clue to the source of the Sun’s energy with his theory of special relativity. One of the implications of this theory is that matter and energy are related by the simple equation:
E = mc2
In other words, a mass (m) can be converted into an amount of energy (E) equivalent to mc2, where c is the speed of light. Because c2 (that is, c × c) is huge, namely, 9 × 1010 km2/s2 (5.6 × 1010 mi2/s2), a small amount of matter can be converted into an awesome amount of energy.
 Inspired by Einstein’s work, physicists discovered that the Sun’s energy output comes from the conversion of matter into energy. In the 1920s, the British astrophysicist Arthur Eddington proposed that the temperature at the center of the Sun, its core, is much greater than had ever been imagined. Calculations eventually revealed that the temperature there is about 15.5 × 106 K. Physicists also showed that at temperatures above about 10 × 106 K, hydrogen fuses into the element helium. In each such reaction, a tiny amount of mass is converted into energy in the form of gamma rays. Enough fusion occurs in its core to account for all of the energy emitted by the Sun.
Inspired by Einstein’s work, physicists discovered that the Sun’s energy output comes from the conversion of matter into energy. In the 1920s, the British astrophysicist Arthur Eddington proposed that the temperature at the center of the Sun, its core, is much greater than had ever been imagined. Calculations eventually revealed that the temperature there is about 15.5 × 106 K. Physicists also showed that at temperatures above about 10 × 106 K, hydrogen fuses into the element helium. In each such reaction, a tiny amount of mass is converted into energy in the form of gamma rays. Enough fusion occurs in its core to account for all of the energy emitted by the Sun.
283
The process of fusing nuclei at such extreme temperatures is called thermonuclear fusion (see Discovery 9-
Hydrogen fusion is also called hydrogen burning, even though nothing is burned in the conventional sense. The ordinary burning of wood, coal, or any flammable substance is a chemical process involving only the electrons orbiting the nuclei of the atoms. Thermonuclear fusion is a far more energetic process that involves violent collisions that change the atomic nuclei themselves. Discovery 9-
 You may have heard statements like “matter is always conserved” or “energy is always conserved.” We know that both of these concepts are incorrect, because mass can be converted into energy, and vice versa. What is true, however, is that the total amount of energy plus mass (multiplied by c2) is conserved. So, the destruction of mass and the creation of energy by the Sun do not violate any laws of nature.
You may have heard statements like “matter is always conserved” or “energy is always conserved.” We know that both of these concepts are incorrect, because mass can be converted into energy, and vice versa. What is true, however, is that the total amount of energy plus mass (multiplied by c2) is conserved. So, the destruction of mass and the creation of energy by the Sun do not violate any laws of nature.
Now let us add up the energy emitted from the entire Sun. As noted earlier in this chapter, the Sun’s mass, usually designated 1 M⊙, is equal to 333,000 Earth masses. Its total energy output per second, called the solar luminosity and denoted 1 L⊙, is 3.9 × 1026 watts. To produce this luminosity, the Sun converts 600 million metric tons of hydrogen into helium within its core each second. This value is twice the mass of the Empire State Building in New York City. This enormous rate is possible because the Sun contains a vast supply of hydrogen—
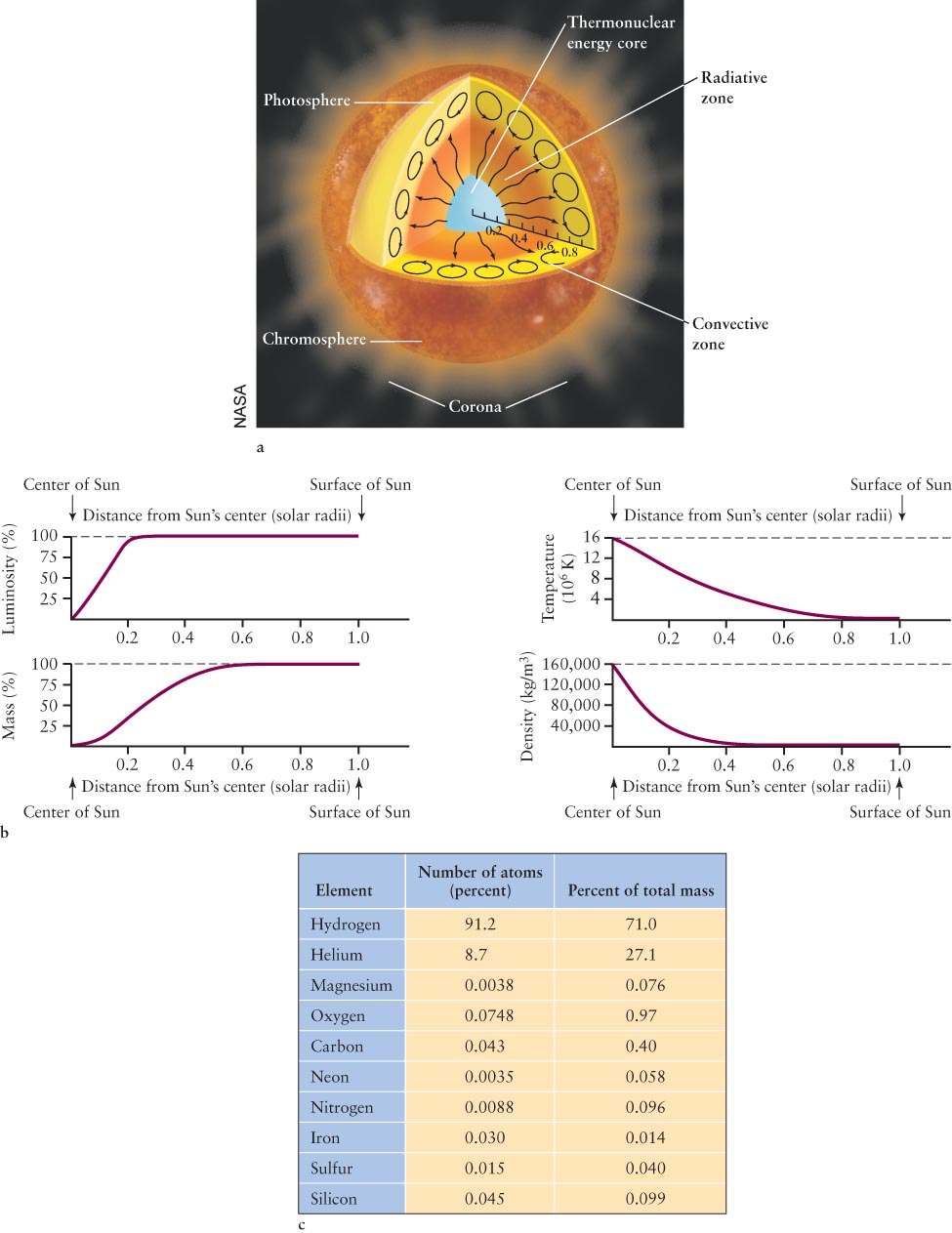
9-8 The solar model describes how energy escapes from the Sun’s core
A scientific theory describing the Sun’s interior, called the solar model, explains how the energy from nuclear fusion in the Sun’s core gets to its photosphere. The model begins with the inward force due to the Sun’s gravity. This force increases the pressure and temperature in the Sun’s core, thereby causing hydrogen fusion to occur there. Because the Sun is not shrinking today, however, there must be an outward force throughout its interior that counters the inward force of gravity. That outward force is created by the gamma-
Consider an idealized journey of one gamma-
Over 1038 fusion events occur every second in the Sun’s core, providing just the right amount of energy to prevent it from collapsing. The balance between the inward force of gravity and the outward force from the motion of the hot gas is called hydrostatic equilibrium (Figure 9-

The outward movement of energy by photons hitting particles, which then bounce off other particles and thereby reemit photons, is called radiative transport, because individual photons are responsible for carrying energy from collision to collision. Calculations show that radiative transport is the dominant means of outward energy flow in the radiative zone, extending from the core to about 70% of the way out to the photosphere.
Focus Question 9-7
Where in the Sun is the energy that we see generated?
Near the photosphere, energy is carried the remaining distance to the surface by the bulk motion of the hot gas, rather than by the flying, energetic photons. As we discussed in Section 7-
284
DISCOVERY 9-1: Thermonuclear Fusion
What drives the thermonuclear fusion that powers the Sun? For nuclei to fuse, they must be brought together at incredibly high temperatures and pressures. That process is exactly what occurs in the Sun’s core, where the entire mass of the Sun compresses inward. The core’s temperature is 15.5 million K, its pressure is about 3.4 × 1011 atm, and its density is 160 times greater than that of water.
285
Normally, nuclei cannot contact one another because the positive electric charge on each proton prevents nearby protons from coming too closely together. (Remember that like charges repel each other.) But in the extreme heat and pressure of the Sun’s center, the protons move so fast in such close proximity that they can stick, or fuse, together.
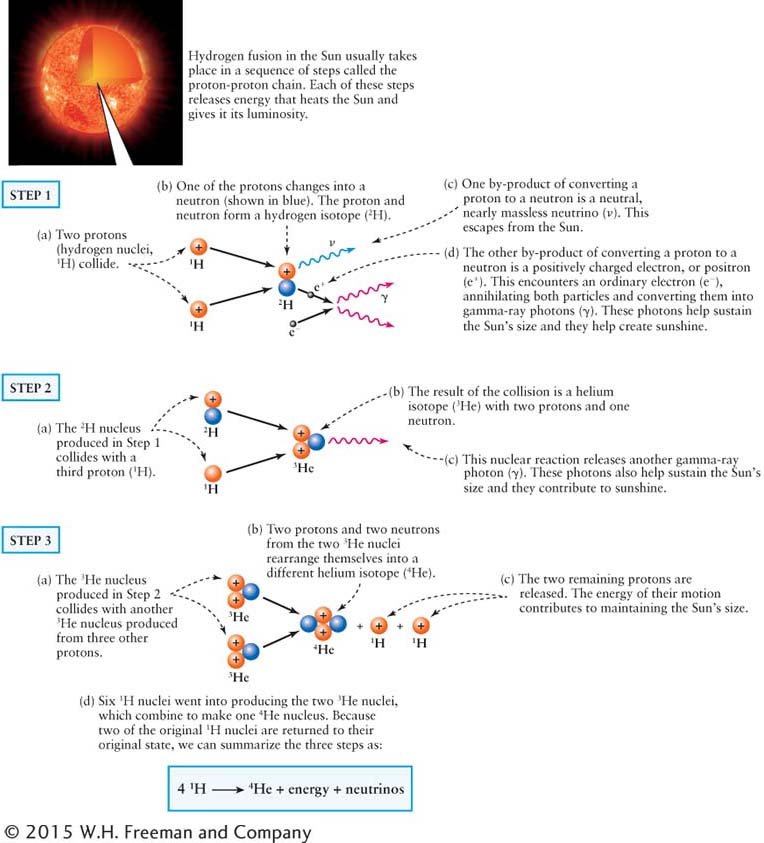
The nuclear transformations inside the Sun follow several routes, but each begins with the simplest atom, hydrogen (H). Most hydrogen nuclei consist of a single proton. The final outcome of fusion is the creation of a nucleus of the next simplest atom, helium (He), consisting of two protons and two neutrons. The fusion of hydrogen into helium takes several steps.
The accompanying diagram on the preceding page shows the most common path for hydrogen fusion in the Sun. This particular sequence is called the proton-
Note that proton-
Because the PP chain produces both neutrinos and the Sun’s energy, we can summarize hydrogen fusion this way:
4 1H → 1 4He + neutrinos + energy
Example: From our summary equation, we can calculate the energy released during a fusion reaction. We simply look at how much mass is converted into energy:
Mass of 4 hydrogen atoms = 6.693 × 10−27 kg
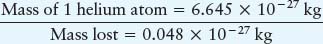
Thus, a small fraction (0.7%) of the mass of the hydrogen going into the nuclear reactions does not show up in the mass of the helium. Ignoring the extremely small mass of the neutrino, this lost mass is converted into energy, as predicted by Einstein’s famous equation:
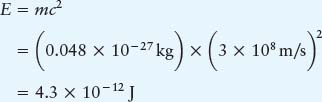
Compare! The energy released from the formation of a single helium atom would light a 9-
286
287
Different gamma rays created in the Sun’s core lose different amounts of energy as they and their successors travel upward through the Sun. Therefore, the photons emitted from the photosphere have a wide range of energies and, hence, wavelengths. The most intense emission is in the visible part of the electromagnetic spectrum. This process of energy loss by photons traveling up from the Sun’s core is the origin of the blackbody nature of the photosphere’s spectrum.
Our model of the Sun’s interior can be expressed as a set of mathematical equations, called the equations of stellar structure. The model quantitatively describes the Sun’s internal characteristics, such as its pressure, temperature, and density at various depths. These equations also describe the conditions inside other stars as they evolve. Because the equations are so complex, astrophysicists today use computers to solve them. Figure 9-
The mass curve rises to nearly 100% at about 0.6 R⊙ from the Sun’s center. Almost all of the Sun’s mass is therefore confined to a volume extending only 60% of the distance from the Sun’s center to its visible surface. This distribution of mass is consistent with the fact that the density of the gas in the photosphere is so low, 104 times lower than the density of the air we breathe.
9-9 The Sun has gotten brighter over time
The Sun fuses hydrogen into helium in its core. Therefore, when the Sun first formed, it had less helium and more hydrogen in its core than it does today. Helium is a denser element than hydrogen and therefore the young Sun’s core was less dense than it is now. Recall that density is the measure of how much mass is packed into any volume. For example, a cubic meter of carbon (with each atom containing 6 protons and 6 neutrons) is less dense than a cubic meter of iron (with each atom containing 26 protons and 30 neutrons).
Focus Question 9-8
Why does the Sun not collapse under the influence of its own enormous gravitational attraction?
When the Sun was young, the larger number of hydrogen atoms (that is, the larger number of protons) in its core created more repulsion than the core experiences today, with its larger number of neutron-
Even though its surface temperature has remained roughly constant, the increase in surface area means that the Sun today is giving off more energy than it formerly emitted. In other words, its luminosity has increased. As more helium accumulated in the core, it became denser and was compressed more by the surrounding mass. This increased compression heated the core to higher temperatures, enabling more fusion to occur. The increased numbers of gamma rays thereby generated were able to push outward more and thereby enlarge the Sun to its present size. The Sun has become brighter, meaning that it is emitting more energy than when it was younger. Calculations indicate that the Sun has gotten about 30% more luminous over the past 4.6 billion years.
Focus Question 9-9
Why did the growing Sun become hotter as measured from Earth if its surface temperature has remained roughly constant?
The fact that the Sun was cooler when the Earth first formed raises the question of whether the Sun always provided enough heat to allow water to be liquid on Earth’s surface. There is overwhelming geological evidence for liquid water back at least 4.2 billion years ago. However, if everything on Earth was then as it is today, the young Sun did not heat Earth sufficiently for its surface water to be liquid. This is called the faint young sun paradox. The paradox is resolved by noting, as we discussed in Section 6-
9-10 The mystery of the missing neutrinos inspired research into the fundamental nature of matter
As explained in Discovery 9-
According to our model of fusion in the Sun, nearly 1038 solar neutrinos per second are produced in the Sun’s core. This output is so huge that here on Earth roughly 100 billion solar neutrinos pass through every square centimeter of your body each second!
288
Occasionally, however, solar neutrinos strike neutrons and convert them into protons. If astronomers could detect even a few of these converted protons, it might be possible to build a “neutrino telescope” that could be used to detect the thermonuclear inferno in the Sun’s core that is hidden from the view of telescopes that collect photons.
Inspired by such possibilities, the American chemist Raymond Davis designed and built a large neutrino detector. This device consisted of a huge tank that contained 100,000 gallons of perchloroethylene (C2Cl4, the fluid your local dry cleaner uses) located deep in the Homestake gold mine in Lead, South Dakota. All neutrino experiments are performed underground to help prevent them from being contaminated by other sources of energy, like high-
On average, solar neutrinos created one radioactive argon atom every 3 days in Davis’s tank. To the consternation of physicists and astronomers, this rate corresponds to only one-
Neutrinos, like the planet Neptune, were discovered because, according to theory, they had to exist. Back in the first few decades of the twentieth century, physicists observed neutrons in nuclei spontaneously transforming into protons and electrons. The transformation is a result of the weak nuclear force, briefly introduced in Section 3-
Among the properties neutrinos had to have are very small masses compared to any other types of particles that have mass and very little interaction with other matter, as we have just discussed. Because electrons or positrons (identical to electrons, except with opposite electric charge) always accompany the formation of the neutrinos created in the Sun, they are often called electron neutrinos.
Two other kinds of neutrinos were subsequently proposed. One, the muon neutrino, is emitted in reactions in which elementary particles, called muons (or antimuons), are released, while the other, the tau neutrino, accompanies the formation of other elementary particles, the tau and antitau particles. The Sun emits neither muon nor tau neutrinos, and the first generation of detectors could only detect electron neutrinos. The solution to the neutrino rate dilemma lay in the existence of muon and tau neutrinos.
Initially, the three “flavors” of neutrinos (electron, muon, and tau) were thought to be massless, and, if so, the equations revealed that they could not change from one flavor into another. But if neutrinos have even the slightest mass, then they can transform from one to another. The transformation of massive neutrinos would occur spontaneously, meaning that if an electron neutrino left the Sun’s core and sped toward Earth, there would be a finite probability (about 65%, actually) that by the time it got here, it would have become a tau or muon neutrino. So, if neutrinos have mass, we should observe only one-
In 1998, the super Kamiokande neutrino detector in Japan observed the other two types of neutrinos coming from the direction of the Sun. The observations were repeated in 2002 at the Sudbury Neutrino Observatory (Figure 9-
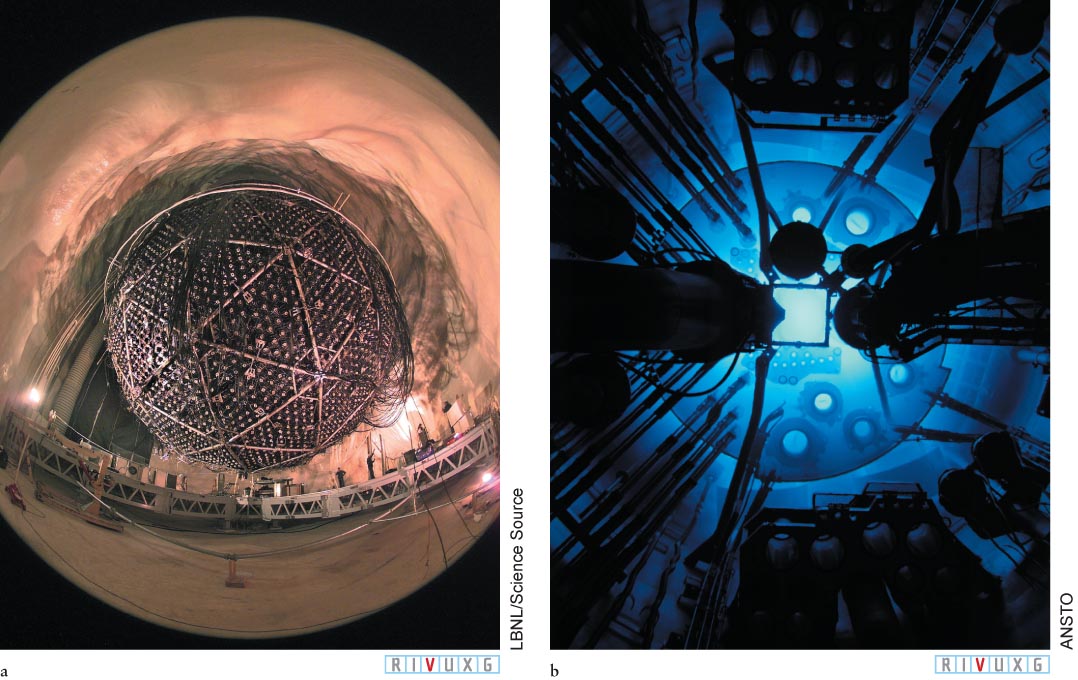

 A Solar Neutrino Experiment (a) Located 2073 m (6800 ft) underground in the Creighton nickel mine in Sudbury, Canada, the Sudbury Neutrino Observatory is centered around a tank that contains 1000 tons of water. Occasionally, a neutrino entering the tank interacts with one or another of the particles already there. Such interactions create flashes of light, called Cerenkov radiation. Some 9600 light detectors sense this light. The numerous silver protrusions are the back sides of the light detectors prior to their being wired and connected to electronics in the lab (seen at the bottom of the photograph). (b) Cerenkov radiation glowing in a nuclear reactor in Australia.
A Solar Neutrino Experiment (a) Located 2073 m (6800 ft) underground in the Creighton nickel mine in Sudbury, Canada, the Sudbury Neutrino Observatory is centered around a tank that contains 1000 tons of water. Occasionally, a neutrino entering the tank interacts with one or another of the particles already there. Such interactions create flashes of light, called Cerenkov radiation. Some 9600 light detectors sense this light. The numerous silver protrusions are the back sides of the light detectors prior to their being wired and connected to electronics in the lab (seen at the bottom of the photograph). (b) Cerenkov radiation glowing in a nuclear reactor in Australia.
To understand how the present generation of neutrino detectors works, let us consider one of the several interactions that occur between neutrinos and other matter. The Sudbury Neutrino Observatory is filled with water that contains a rare type of hydrogen nucleus, called deuterium, which consists of a proton and a neutron. When a solar neutrino is absorbed by a deuterium nucleus, the nucleus breaks apart into two protons and an electron. As this electron rushes through the water, it emits a flash of light, called Cerenkov radiation (Figure 9-
289
As the Russian physicist Pavel A. Cerenkov (pronounced Che-
Thus, scientists detect neutrinos by observing Cerenkov radiation flashes with light-