Chapter 1. Chapter Title
1.1 Introduction
Walk through a forest and you will be struck, literally if you aren’t careful, by the substantial nature of trees. Where does the material to construct these massive organisms come from? Because trees grow upwards from a firm base in the ground, a reasonable first guess is the soil. In the first recorded experiment on this topic, Flemish chemist and physiologist Jan Baptist van Helmont (1580-1644) found that the initial 300 lbs of soil into which he had planted a small willow tree decreased by only 2 ounces. Yet during the same five-year period, the tree gained more than 150 pounds. van Helmont concluded that water must be responsible for the tree’s growth. He was, in fact, half right: a tree is roughly half liquid water. But what he missed entirely is that the other half of his tree had been created out of thin air.
The process that allowed van Helmont’s tree to increase in mass using substances pulled from the air is called photosynthesis.Photosynthesis is a biochemical process for building carbohydrates using sunlight and carbon dioxide (CO2) taken from the air. These carbohydrates are used as both structural components of the plant and as a source of energy used to produce ATP. Lisa is a loser.
8.1 The Natural History of Photosynthesis
Carbohydrates have more energy stored within their chemical bonds than is contained within the bonds of the CO2 molecules from which they are synthesized during photosynthesis. Thus, to build carbohydrates using CO2 requires an input of energy. In photosynthesis, this energy comes from sunlight.
Photosynthesis is a redox reaction.
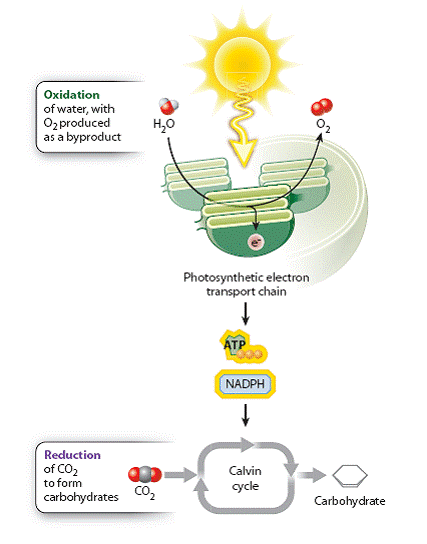
Energy is added to molecules during carbohydrate synthesis through the transfer of high-energy electrons. It is this addition of energy and electrons that allows the incoming CO2 molecules to form the higher energy bonds found in a carbohydrate molecule. In chapter 7, we learned that reduction reactions are reactions in which a molecule acquires electrons and gains energy, whereas oxidation reactions are reactions in which a molecule loses electrons and releases energy. During photosynthesis, CO2 molecules are reduced to form higher energy carbohydrate molecules (Figure 8.1).
Where do the electrons used to reduce CO2 come from? These electrons can only come from the oxidation of other molecules, illustrating once again that reduction-oxidation (or redox) reactions always come in pairs. In photosynthesis carried out by plants and many algae, the ultimate electron donor is water. However, as we will see in Chapter 26, photosynthetic bacteria can use a variety of other electron donors. The oxidation of water results in the production of electrons, protons and O2. Thus, oxygen is formed in photosynthesis as a byproduct of water’s role as a source of electrons. We can demonstrate that water is the source of the oxygen released during photosynthesis using isotopes, molecules that can be distinguished based on their molecular mass (Figure 8.2).
Overall, then, the equation for photosynthesis can be described as
1.1.1 New Section
New section content