Appendix D: Temperature Scales
Three temperature scales are in common use. Throughout most of the world, temperatures are expressed in degrees Celsius (°C), named in honor of the Swedish astronomer Anders Celsius, who proposed it in 1742. The Celsius temperature scale (also known as the “centigrade scale”) is based on the behavior of water, which freezes at 0°C and boils at 100°C at sea level on Earth.
Scientists usually prefer the Kelvin scale, named after the British physicist Lord Kelvin (William Thomson), who made many important contributions to our knowledge about heat and temperature. On the Kelvin temperature scale, water freezes at 273 K and boils at 373 K. Note that we do not use the degree symbol with the Kelvin temperature scale.
Because water must be heated by 100 K or 100°C to go from its freezing point to its boiling point, you can see that the size of a kelvin is the same as the size of a degree Celsius. When considering temperature changes, measurements in kelvins and in degrees Celsius lead to the same number.
A temperature expressed in kelvins is always equal to the temperature in degrees Celsius plus 273. Scientists prefer the Kelvin scale because it is closely related to the physical meaning of temperature. All substances are made of atoms, which are very tiny (a typical atom has a diameter of about 10−10 m) and constantly in motion. The temperature of a substance is directly related to the average speed of its atoms. If something is hot, its atoms are moving at high speeds. If a substance is cold, its atoms are moving much more slowly.
The coldest possible temperature is the temperature at which atoms move as slowly as possible (they can never quite stop completely). This minimum possible temperature, called absolute zero, is the starting point for the Kelvin scale. Absolute zero is 0 K, or −273°C. Because it is impossible for anything to be colder than 0 K, there are no negative temperatures on the Kelvin scale.
A-6
In the United States, many people still use the now outdated Fahrenheit scale, which expresses temperature in degrees Fahrenheit (°F). When the German physicist Gabriel Fahrenheit introduced this scale in the early 1700s, he intended 0°F to represent the coldest temperature then achievable (with a mixture of ice and salt water) and 100°F to represent the temperature of a healthy human body. On the Fahrenheit scale, water freezes at 32°F and boils at 212°F. Because there are 180 degrees Fahrenheit between the freezing and boiling points of water, a degree Fahrenheit is only 100/180 (= 5/9) the size of the other scales.
The following equation converts from degrees Fahrenheit to degrees Celsius:
TC = 5/9 (TF − 32)
To convert from Celsius to Fahrenheit, a simple rearrangement of terms gives the relationship
TF = 9/5 TC + 32
where TF is the temperature in degrees Fahrenheit and TC is the temperature in degrees Celsius.
For example, consider a typical room temperature of 68°F. Using the first equation, we can convert this measurement to the Celsius scale as follows:
TC = 5/9 (68 − 32) = 20°C
To arrive at the Kelvin scale, we simply add 273 degrees to the value in degrees Celsius. Thus, 68°F = 20°C = 293 K. The accompanying figure displays the relationships among these three temperature scales.
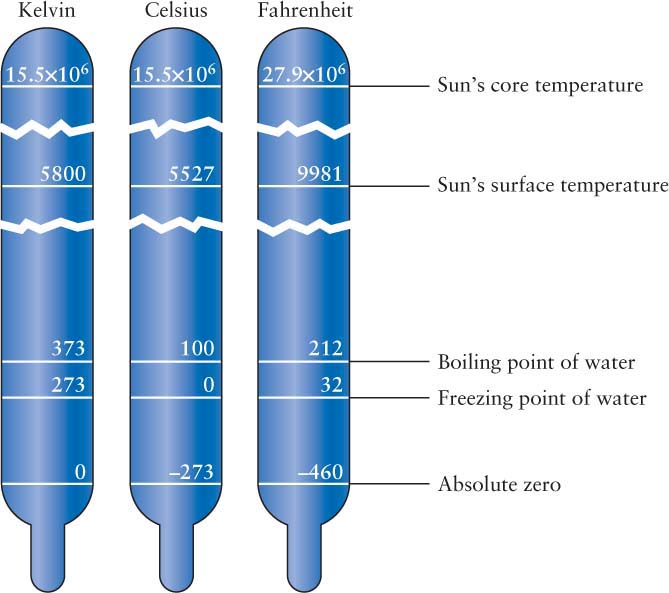
Try these questions: The Sun’s surface temperature is about 5800 K. What is its temperature in Celsius and Fahrenheit? The temperature of empty space is about 3 K. What is its temperature in Celsius and Fahrenheit?
(Answers appear at the end of the book.)
A-7