The existence of life depends on chemical and physical properties of matter
 To build a physical foundation for astrobiology, let us begin by considering some of the fundamental requirements for the existence of life as we know it. First is the presence of liquid water. Water, which is neither acidic nor basic, enables many kinds of atoms and molecules dissolved in it to bond to one another or separate from one another in large numbers. While water molecules combine with some atoms and molecules, water also allows many interactions to occur in it without chemical interference. Such diverse activity is essential for the creation and evolution of the complex molecules and systems of molecules that function in living organisms. Given all of these factors, biologists believe that life on Earth began to develop in primordial bodies of water.
To build a physical foundation for astrobiology, let us begin by considering some of the fundamental requirements for the existence of life as we know it. First is the presence of liquid water. Water, which is neither acidic nor basic, enables many kinds of atoms and molecules dissolved in it to bond to one another or separate from one another in large numbers. While water molecules combine with some atoms and molecules, water also allows many interactions to occur in it without chemical interference. Such diverse activity is essential for the creation and evolution of the complex molecules and systems of molecules that function in living organisms. Given all of these factors, biologists believe that life on Earth began to develop in primordial bodies of water.
The second requirement is an element that can bond strongly, but not too strongly, with at least three other atoms to allow for complex molecules necessary for life. Atoms able to make only two bonds can form chains (Figure 19-2a), or loops of atoms, but cannot create complex structures (Figure 19-2b) that contain atoms of other elements, as is necessary for making the variety of molecules essential for life.
Five elements can bond covalently (that is, by sharing electrons) with three or more elements: boron (B), carbon (C), nitrogen (N), silicon (Si), and phosphorus (P). Bonding by donating or receiving electrons (called ionic bonding) creates bonds that are unsuitable for the formation of the complex, flexible, rapidly changing molecules that life requires. Carbon is unique among the five elements that can make at least three covalent bonds in that its bonds are flexible yet strong. As counter-examples, silicon-silicon bonds come apart under the slightest disturbance; silicon-oxygen bonds create gels and liquids that are very hard to alter (Figure 19-3a); and silicon-oxygen-oxygen bonds are so strong that they create rocks (Figure 19-3b). The other three elements that might serve as the backbone of life—boron, nitrogen, and phosphorus—have similar bonding problems. Despite the enormous range of conditions under which terrestrial life exists, all of it is based on the unique properties of carbon.

 Carbon atoms form chemical bonds that can combine to create especially long, complex molecules. These molecules can be further linked in elaborate chains, lattices, and fibers. It is for these reasons that carbon-based compounds, called organic molecules, are the stuff of life. Indeed, biologists have already identified more than 6 million carbon compounds.
Carbon atoms form chemical bonds that can combine to create especially long, complex molecules. These molecules can be further linked in elaborate chains, lattices, and fibers. It is for these reasons that carbon-based compounds, called organic molecules, are the stuff of life. Indeed, biologists have already identified more than 6 million carbon compounds.
560
The variety and stability of living organisms depend on the complex, self-regulating, chemical reactions of organic molecules. Happily, carbon is also among the most abundant elements in the universe. The unique versatility and abundance of carbon imply that extraterrestrial biology would also be based on organic chemistry.
Third, a life-supporting environment must also have a variety of other elements essential for developing life. Other constituents of organic molecules, such as hydrogen, oxygen, nitrogen, and sulfur, are plentiful in our Galaxy. All of the organic molecules necessary to form life may develop naturally in the oceans of Earth and similar planets. In order to expedite the enrichment of oceans with the primordial soup of elements necessary for life, it may be necessary for Earthlike worlds to have large moons, like ours. When they are young and close to their planets (remember from Chapter 6 that our Moon was much closer and is still spiraling away), such moons create powerful tides that can quickly erode the continental shorelines and supply large quantities of minerals to the oceans.
Another way that the formation of life may have been expedited on some worlds is from organic material from space that rained down on them from space debris. This belief is supported by the fact that spectra taken of comets in our solar system reveal that they contain an assortment of organic compounds. Further evidence for the existence of organic molecules in interplanetary space was collected by, among others, the spacecraft Giotto as it passed close to the nucleus of Halley’s comet. In 1997, NASA launched the Stardust mission, which rendezvoused with Comet Wild 2 in January 2004, collecting dust and other debris from the comet. The spacecraft returned safely to Earth in January 2006. It revealed the presence of organic molecules that had been released from the comet, supporting the belief that comets may have helped seed compounds useful in the formation of life on Earth.
Evidence of extraterrestrial organic molecules also comes from certain meteorites called carbonaceous chondrites (Figure 19-4). Many of the carbonaceous chondrites that have fallen to Earth contain a variety of organic substances. As noted in Section 9-11, carbonaceous chondrites are ancient meteorites dating from the formation of the solar system. If they contained organic molecules while in space, it seems reasonable to conclude that, even from their earliest days, the planets have been continually bombarded with organic compounds.

In interstellar clouds, carbon atoms have combined with other elements to produce an impressive variety of organic compounds. Since the 1960s, radio astronomers have detected telltale microwave emission lines from interstellar clouds that help identify more than 80 of these carbon-based compounds. Examples include polycyclic aromatic hydrocarbons (PAHs), a type of molecule composed of just carbon and hydrogen atoms, as well as ethyl alcohol (CH3CH2OH), formaldehyde (H2CO), acetic acid (HCOOCH3), and acetaldehyde (CH3CHO). In 2003, scientists detected the amino acid glycine in space. In 2010, molecules with 60 carbon atoms, called buckyballs because they look like structures designed by Buckminster Fuller, were observed in a planetary nebula. In 2005, PAHs were observed more than 9 billion light-years away. These were the first observations of complex organic molecules from that far in the past. Because stars and planets condense from interstellar gas and dust enriched in these elements, these compounds can help provide the building blocks of life elsewhere in the Galaxy and, indeed, in many other galaxies throughout the universe. Indeed, in 2004, astronomers using the Spitzer Space Telescope detected some of the ingredients for life, including methanol, water, and carbon dioxide, in orbit around newly forming stars.
561
Interstellar space is not the only source of organic material. In a classic experiment performed in 1952, the American chemists Stanley Miller (1930–2007) and Harold Urey (1893–1981) demonstrated that simple chemicals can combine on liquid-water–bearing planets to form organic compounds. Hoping to recreate conditions on primitive Earth and in its atmosphere, as then understood, Miller and Urey mixed hydrogen, ammonia, methane, and water vapor in a closed container. They then subjected their mixture to an electric arc to simulate lightning bolts. At the end of a week, the inside of the container had become coated with a reddish-brown substance rich in organic compounds essential to life (Figure 19-5).
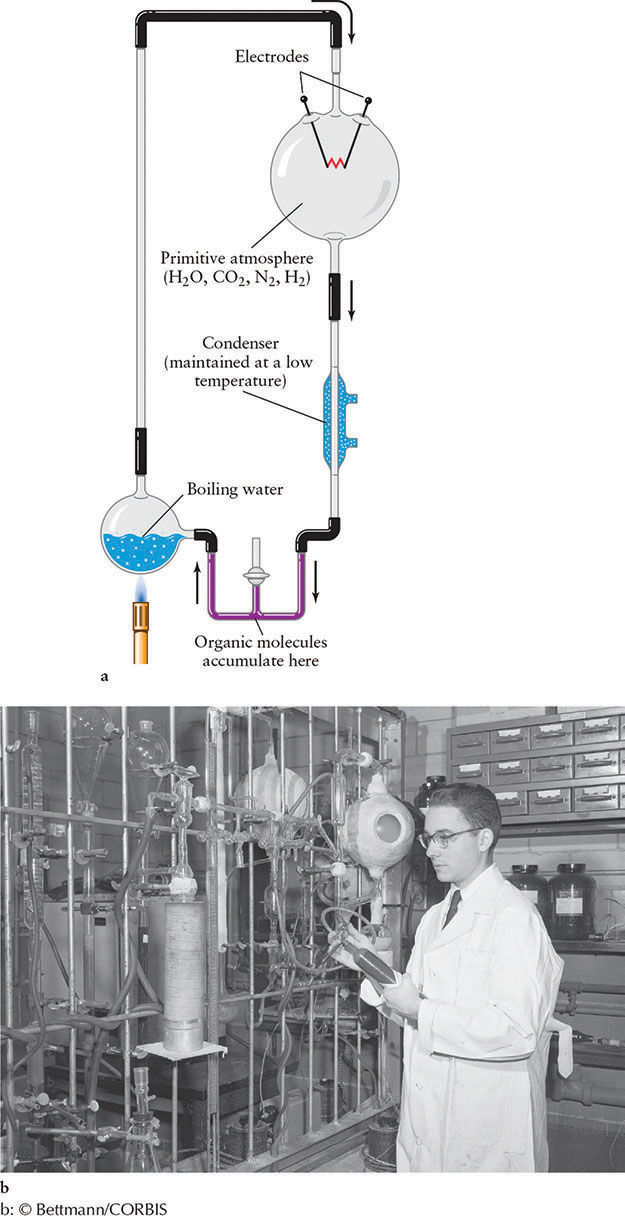
Since that time, geochemists have come to realize that Earth’s early atmosphere was more likely to have been a mixture of carbon dioxide, nitrogen, and hydrogen, along with water vapor “outgassed” from volcanoes and deposited by comet impacts. Modern versions of the Miller-Urey experiment have therefore used these common gases, and, once again, organic compounds were produced.
Keep in mind that scientists have not yet created life in a test tube. Biologists have yet to figure out, among other things, how simple organic molecules gathered into cells and developed systems for self-replication.
562
The fourth issue surrounding the existence of life elsewhere relates to physical properties of planets—the environments supporting life-forms must have suitable temperature, radiation, and other factors. The fundamental issue here is that water must be liquid on a world for surface life to evolve there. The region around a star where water can be liquid on a planet is called the star’s habitable zone.
If a planet is too close to a star, the temperature and deadly ultraviolet radiation levels will be too high for life to evolve very far. If the planet is too far away, it will be too cold to have liquid water. Earth is in the Sun’s habitable zone, while Venus is not.
If a planet’s environment is otherwise hostile—such as having too much radiation, being too windy, or being too seismically active—life may either not be able to spread or may quickly become extinct. If the star is too massive, it will explode before advanced life on any of its planets has a chance to evolve very far. If the star is too small, the planet would have to be very close to it to be warm enough for life, but then tidal forces from the star would lock the planet in synchronous rotation, making most of its surface either too hot (daytime side) or too cold (nighttime side) for life to flourish. Even a world in synchronous rotation with a suitable temperature on the star-facing side is very unlikely to support life, because water in its atmosphere will drift to the night side, permanently freeze, and the star-lit side will eventually become arid. These issues are summarized in Figure 19-6.

 Zone For Habitable Planets This figure summarizes the locations in the Galaxy and in orbit around stars where habitable planets might be found. Earth, of course, is in such a location.
Zone For Habitable Planets This figure summarizes the locations in the Galaxy and in orbit around stars where habitable planets might be found. Earth, of course, is in such a location.
Margin Question 19-2
Question
Synchronous rotation occurred in what other situation that we explored earlier in this book?
Margin Question 19-3
Question
How is water inside worlds like Europa kept liquid?
Under the right conditions, moons also have the potential for developing and supporting life. In the habitable zones of stars, sufficiently massive moons (such as Earth-sized terrestrial moons of giant planets in these regions) could sustain liquid water on their surfaces. Even less massive moons could have water below their surfaces kept in the liquid state. This would occur if they orbit their planets in sufficiently elliptical orbits so that changes in the tidal forces from their planets would continually change their shape and thereby warm their insides, as discussed in Chapter 8 for Europa and Ganymede orbiting Jupiter. Such moons would not have to be in the habitable zones of their stars.