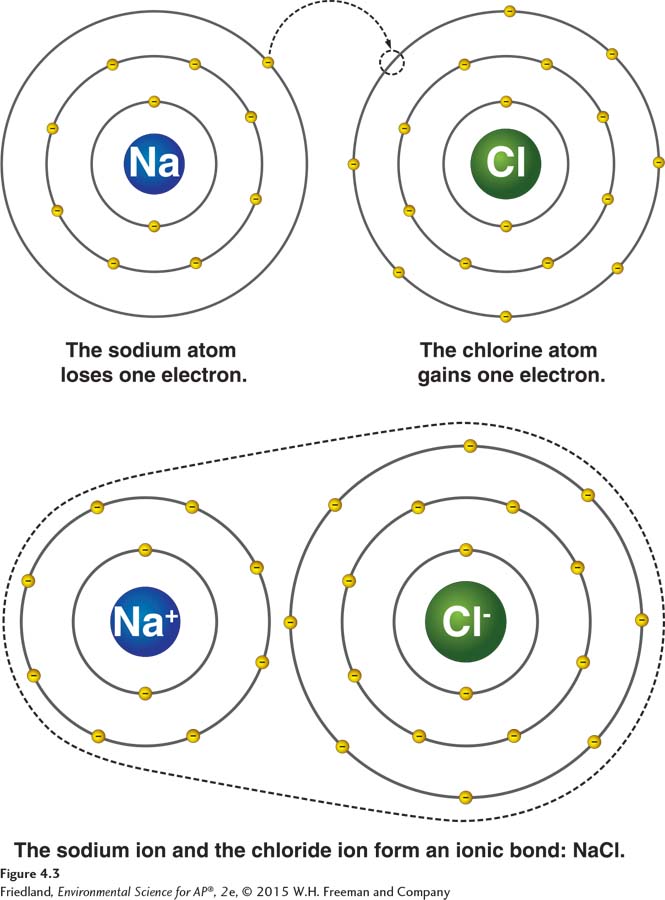
FIGURE 4.3 Ionic bonds. To form an ionic bond, the sodium atom loses an electron and the chlorine atom gains one. As a result, the sodium atom becomes a positively charged ion (Na+) and the chlorine atom becomes a negatively charged ion (Cl−, known as chloride). The attraction between ions of opposite charges— d—