module 35 Fossil Fuel Resources
We have seen that fossil fuels provide most of the commercial energy in the world. These fuels are also responsible for a good deal of the pollution that occurs, a topic that will be discussed in Chapter 15. Because fossil fuels are such an important energy source, environmental scientists must study them closely. In this module, we will examine the major fossil fuels—
Learning Objectives
After reading this module, you should be able to
discuss the uses of coal and its consequences.
discuss the uses of petroleum and its consequences.
discuss the uses of natural gas and its consequences.
discuss the uses of oil sands and liquefied coal and their consequences.
describe future prospects for fossil fuel use.
Coal is the most abundant and dirtiest of the fossil fuels
Coal A solid fuel formed primarily from the remains of trees, ferns, and other plant materials preserved 280 million to 360 million years ago.
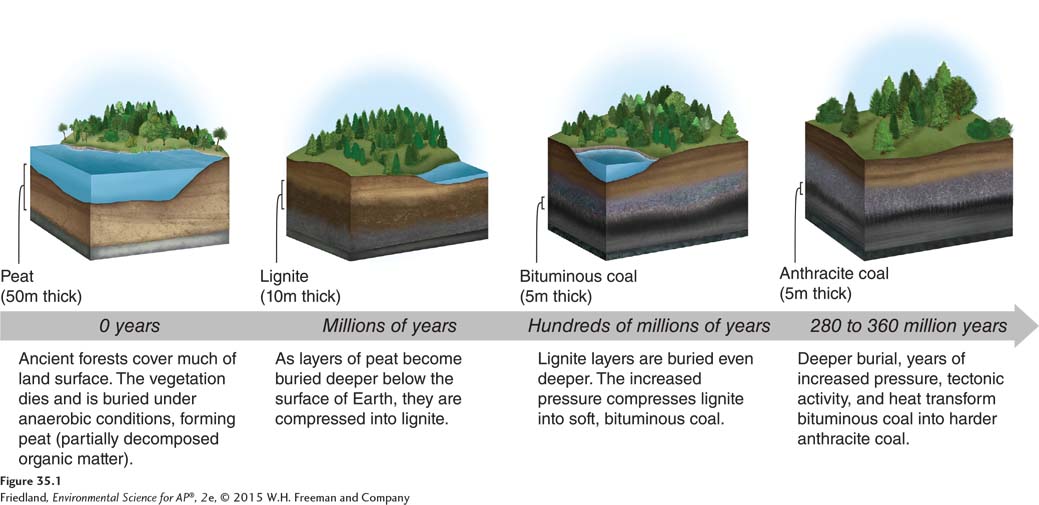
Coal is a solid fuel formed primarily from the remains of trees, ferns, and other plant materials that were preserved 280 million to 360 million years ago. Coal has been the “work horse” of fossil fuels in the United States and in many other parts of the world. It is abundant in many areas and often is relatively easy to extract, handle, and process. We have seen that coal is the fuel most commonly used for electricity generation in the United States. There are three types of coal, ranked from lesser to greater age, exposure to pressure, and energy content; they are lignite, bituminous, and anthracite. A precursor to coal, called peat, is made up of partly decomposed organic material, including mosses. The formation of coal takes hundreds of millions of years. FIGURE 35.1 represents factors involved in the formation of the different types of coal. Starting with an organic material such as peat, increasing time and pressure produce successively denser coal with more carbon molecules, and more potential energy, per kilogram.
The largest coal reserves are found in the United States, Russia, China, and India. The countries that are currently producing the greatest amounts of coal are China, the United States, India, and Australia.
Advantages of Coal
Because it is energy-
Disadvantages of Coal
Although coal is a relatively inexpensive fossil fuel, its use does have several disadvantages. As we saw in Chapter 8, the environmental consequences of the tailings from surface mining are significant. As surface coal is used up and becomes harder to find, however, subsurface mining becomes necessary. With subsurface mining, the technological demands and costs increase, as do the consequences for human health.
Coal contains a number of impurities, including sulfur, that are released into the atmosphere when the coal is burned. The sulfur content of coal typically ranges from 0.4 to 4 percent by weight. Lignite and anthracite have relatively low sulfur contents, whereas the sulfur content for bituminous coal is often much higher. Trace metals such as mercury, lead, and arsenic are also found in coal. Combustion of coal results in the release of these impurities, which leads to an increase of sulfur dioxide and other air pollutants, such as particulates, in the atmosphere, as we will see in Chapter 15. Compounds that are not released into the atmosphere remain behind in the resulting ash.
In order to reduce the chemical compounds released into the air, coal companies wash their coal in a variety of organic compounds. In some cases, these compounds have not been widely tested for toxicity or their effect on humans and ecosystems. In January 2014, a chemical storage tank containing one such coal cleaning compound leaked in West Virginia. Residents nearby immediately noticed a sweet odor and ultimately over 300,000 residents were advised not to drink their water for a number of days. Unfortunately, chemical spills are not the only accidents related to coal combustion. The residual ash from coal combustion can also be a problem.

According to the U.S. Department of Energy, there are 1,450 coal mines in the United States and they produced roughly 1 billion metric tons of coal in 2012. Most of that coal is burned in the United States, with anywhere from 3 to 20 percent of it remaining behind as ash. Large deposits of this ash are often stored near coal-
Finally, coal is a significant source of air pollution. Coal is 60 to 80 percent carbon. When it is burned, most of that carbon is converted into CO2 and energy is released in the process. Coal produces far more CO2 per unit of energy released than either oil or natural gas and it contributes to the increasing atmospheric concentrations of CO2.
Petroleum is cleaner than coal
Petroleum A fossil fuel that occurs in underground deposits, composed of a liquid mixture of hydrocarbons, water, and sulfur.
Petroleum, another widely used fossil fuel, is a fluid mixture of hydrocarbons, water, and sulfur that occurs in underground deposits. While coal is ideal for stationary combustion applications such as those in power plants and industry, the fluid nature of petroleum products such as oil and gasoline makes them more suitable for mobile combustion applications, such as in vehicles.
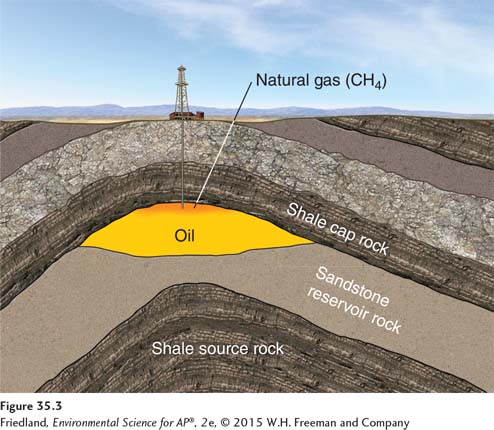
Petroleum is formed from the remains of ocean-
Petroleum contains natural gas. As we can see from FIGURE 35.3, some of this gas separates out naturally. If you have ever seen a burning flame in photographs of oil wells, it is a gas flare created when oil workers flare, or burn off, the natural gas, which is done under controlled conditions to prevent an explosion. As we will see in the next section, some of the gas is also extracted for use as fuel.
Crude oil Liquid petroleum removed from the ground.
Liquid petroleum that is removed from the ground is known as crude oil. The U.S. Department of Energy refers to oil, crude oil, and petroleum as equivalent substances, and we will do the same in this chapter. Crude oil can be further refined into a variety of compounds. These compounds, including tar and asphalt, gasoline, diesel, and kerosene, are distinguished by the temperature at which they boil and can therefore be separated by heating the petroleum. This process takes place in an oil refinery, a large factory of the kind shown in this chapter’s opening photograph. The refining process is complex and dangerous, and requires a major financial investment. There are roughly 150 oil refineries in the United States; some of the larger ones can refine over 80 million liters (21 million gallons) per day. Oil production and sales are measured in barrels of oil; one barrel equals 160 L (42 gallons).
As we saw in FIGURE 34.4a, the United States uses more petroleum than any other fuel—
The top petroleum-
Advantages of Petroleum
Because petroleum is a liquid, it is extremely convenient to transport and use. It is relatively energy-
Disadvantages of Petroleum
Oil, like coal, contains sulfur and trace metals such as mercury, lead, and arsenic, which are released into the atmosphere when it is burned. Some sulfur can be removed during the refining process, so it is possible, though more expensive, to obtain low-

As we have seen, oil must be extracted from under the ground or beneath the ocean. Whenever oil is extracted and transported, there is the potential for oil to leak from the wellhead or to be spilled from a pipeline or tanker. Some oil naturally escapes from the rock in which it was stored and seeps into water or out onto land. However, commercial oil extraction has greatly increased the number of leakage and spillage events and the amount of oil that has been lost to land and water around the world. As noted at the beginning of the chapter, the largest oil spill in the United States until 2010 was the Exxon Valdez oil tanker accident in 1989. More recently, the blowout of the BP Deepwater Horizon oil well, drilled 81 km (50 miles) off the coast of Louisiana in 1,524 m (5,000 feet) of water, led to a spill of well over 780 million liters (206 million gallons) of oil (FIGURE 35.4).
Larger oil spills have occurred elsewhere in the world. For example, during the 1991 Persian Gulf War, approximately 912 million liters (240 million gallons) of petroleum were spilled when wellheads were deliberately sabotaged or destroyed by the Iraqi army in Kuwait.
Oil is spilled into the natural environment in various ways. A 2003 National Academy of Sciences study found that oil extraction and transportation were responsible for a relatively small fraction of the oil spilled into marine waters worldwide. It found that roughly 85 percent of the oil entering marine waterways came from runoff from land and rivers, airplanes, and small boats and personal watercraft, including both deliberate and accidental releases of waste oil.
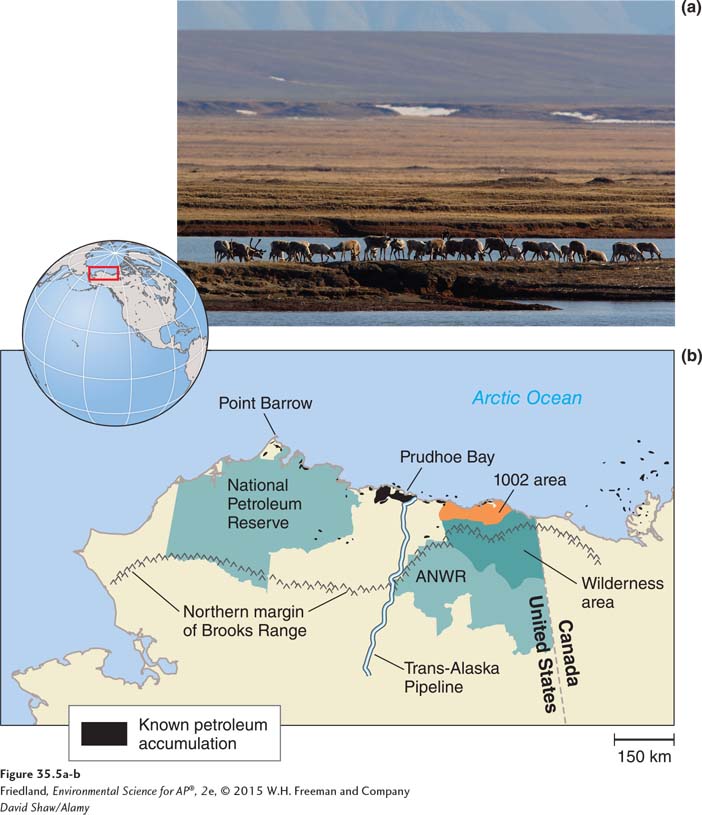
In the United States, debates continue over the trade off between domestic oil extraction and the consequences for habitat and species living near oil wells or pipelines. For example, when a 1,300-
The debate about the environmental effects of land-
Humans, as well as wildlife, have been harmed by oil extraction. In Nigeria and many other developing countries, oil fields are adjacent to villages. Thick, gelatinous crude oil covers the ground where people walk, sometimes in bare feet. Oil flaring—
Natural gas is the cleanest of the fossil fuels
We have already mentioned natural gas in connection with petroleum, since it exists as a component of petroleum in the ground as well as in gaseous deposits found separately from petroleum. Natural gas is 80 to 95 percent methane (CH4) and 5 to 20 percent ethane, propane, and butane. Because natural gas is lighter than oil, it lies above oil in petroleum deposits (see FIGURE 35.3). Natural gas is generally extracted in association with petroleum; only recently has exploration specifically for natural gas been conducted.
The two largest uses of natural gas in the United States are for electricity generation and industrial processes. Natural gas is also used to manufacture nitrogen fertilizer, and it is used in homes as an efficient fuel for cooking, heating, and operating clothes dryers and water heaters. Compressed natural gas can be used as a fuel for vehicles, but because it must be transported by pipeline, it is not accessible in all parts of the United States and is therefore unlikely to become an important fuel for cars. Liquefied petroleum gas (LPG)—which is similar to natural gas, but in a liquid form—
Advantages of Natural Gas
Because of the extensive natural gas pipeline system in many parts of the United States, roughly one-

Disadvantages of Natural Gas
While natural gas when combusted releases the least carbon dioxide of all the fossil fuels, unburned natural gas—
The process of exploring for natural gas involves the “thumper trucks” already mentioned. As described in Chapter 1, the process of hydraulic fracturing (fracking) releases natural gas from host rock. Fracking uses large amounts of chemicals and because those chemicals do not need to be named, their effects are unknown. Fracking also uses large quantities of water that can become contaminated and must be disposed of afterward. There can be groundwater contamination resulting from the drilling of natural gas wells. As hydraulic fracturing has grown more widespread, a number of scientists and gas extraction experts have attempted to quantify exactly how much natural gas escapes during the extraction and transportation process. Estimating the amount of escaped natural gas has become a controversial subject due to the large uncertainties in the natural gas extraction process, measurement difficulties, and industrial secrecy. Presently, estimates of escaped gas range from 2 to 9 percent of the total amount of gas extracted.
Oil sands and liquefied coal are also fossil fuels
Other types of fossil fuel deposits contain a great deal of energy, but are not readily available. When we consider using these energy resources, it is important to determine the energy return on energy investment. Two of the less readily available fossil fuels are oil sands and liquid coal.
Oil Sands
Oil sands Slow-
Bitumen A degraded petroleum that forms when petroleum migrates to the surface of Earth and is modified by bacteria.
Not all petroleum is easily extractable in conventional oil wells. Oil sands are slow-
Although oil sand exploitation promises to extend our petroleum supply, it could have serious negative environmental impacts. The mining of bitumen is much more energy-
Liquid Coal
CTL (coal to liquid) The process of converting solid coal into liquid fuel.
Whereas the availability of petroleum may become much more limited in this century as supplies diminish, the United States and China both have immense coal reserves. The technology to convert solid coal into a liquid fuel—
Fossil fuels are a finite resource
Energy intensity The energy use per unit of gross domestic product.
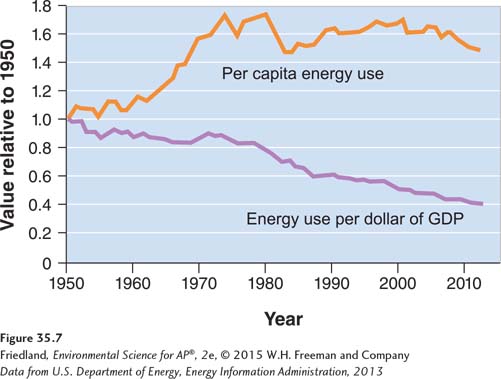
Although we know that the supply of fossil fuels is finite, there is some discussion within the environmental science community on whether or not that matters. Recall our discussion in Chapter 7 about human creativity. Many people believe that we will apply human ingenuity to develop new energy sources. In the meantime, total energy use has leveled off, and energy use per person has decreased slightly. In addition, energy use per unit of gross domestic product (GDP), known as energy intensity, has been steadily decreasing, as FIGURE 35.7 shows. In other words, we are using energy more efficiently, but because the human population has grown so much and we are doing more things that use energy, our overall energy use has not decreased. For example, think about how many electronic devices you have, then ask your parents or grandparents how many electronic devices they had when they were high school students. We will begin this section with a survey of ideas about the availability of fuel and its use over time, and then we will look at possible patterns of fossil fuel energy use in the future.
The Hubbert Curve
We have seen that fossil fuels, our primary source of energy, are finite. Because fossil fuels take millions of years to form, they are nonrenewable resources, at least on a human timescale. By definition, then, the use of fossil fuels is not sustainable because there is no way to limit our consumption to the rate at which these fuels are being formed. Experts have debated whether our economy will at some point be limited by the availability of this energy resource, although in recent years, concerns have shifted away from the actual supply of fossil fuels to the consequences of fossil fuel combustion, particularly the release of CO2 and its contribution to global warming. Many environmental scientists believe that these consequences will manifest themselves in adverse ways long before we run out of fossil fuels. However, there is still a group of energy analysts deeply concerned about “the end of oil.”
Hubbert curve A bell-
Peak oil The point at which half the total known oil supply is used up.
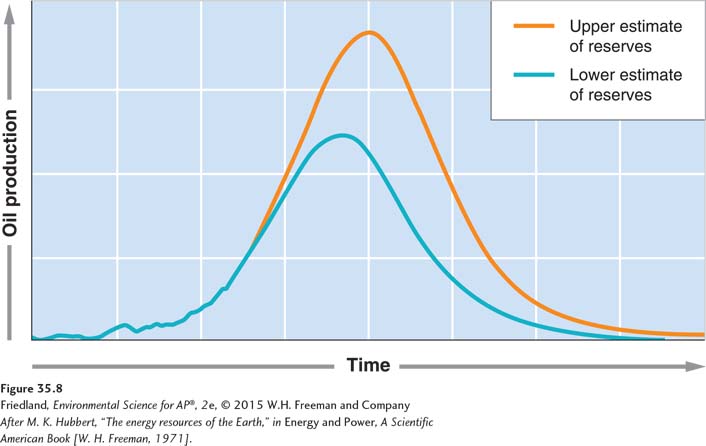
In 1969, M. King Hubbert, a geophysicist and oil company employee, published a graph showing a bell-
Although there have been discoveries of large oil fields since Hubbert did his work, the conclusion he drew from his model still holds. Regardless of the exact amount of the total reserves, the total number of years we use petroleum will fall within a relatively narrow time window. When we identify a fuel source, we tend to use it until we come upon a better fuel source. As a number of energy experts are fond of saying, “We did not move on from the Stone Age because we ran out of stones.” In a similar vein, many people believe that ingenuity and technological advances in the renewable energy sector will one day render oil much less desirable. A growing number of energy analysts maintain that wondering about how much oil remains and whether or not we have reached peak oil are no longer meaningful questions to ask. As the undesirability of fossil fuels becomes greater they ask, “What fuel will we use next?”
The Future of Fossil Fuel Use
If current global use patterns continue, and no significant additional oil supplies are discovered, we may run out of conventional oil supplies in less than 50 years. Natural gas supplies will last longer. Coal supplies will last for at least 200 years, and probably much longer. While these predictions assume that we will continue our current use patterns, advances in technology, a shift to nonfossil fuels, or changes in social choices and population patterns could alter them.
As people have come to accept the theory that anthropogenic increases in atmospheric greenhouse gas concentrations have caused global climate change, a large number of researchers have suggested that we should turn our attention to how we might transition from fossil fuels before their use causes further problems.
Concerns about scarcity, environmental impacts (especially the influence of CO2 on global climate change), and rising costs of fossil fuels present many opportunities. Rising oil prices create a powerful incentive to invest in alternative energy resources and conservation. As we will see in Chapter 13, people have begun to explore the possibility of a new infrastructure to deliver wind energy, hydroelectricity, and solar energy. On the other hand, the higher price of oil makes formerly unproductive mining and extraction methods cost-