module 36 Nuclear Energy Resources
Because the combustion of fossil fuels releases large quantities of CO2 into the atmosphere, nuclear energy has received increasing interest. Concerns about nuclear energy include radioactivity, the proliferation of radioactive fuels that could be used in weapons, and the potential for accidents. Recently, however, nuclear energy has received positive attention, even from self-
Learning Objectives
After reading this module, you should be able to
describe how nuclear energy is used to generate electricity.
discuss the advantages and disadvantages of using nuclear fuels to generate electricity.
Nuclear reactors use fission to generate electricity
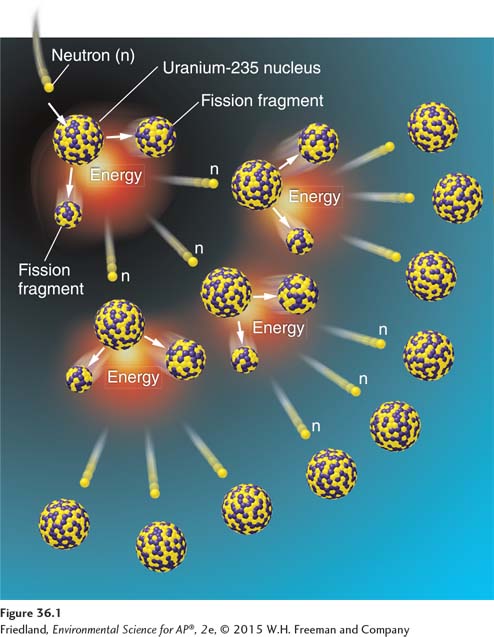
Electricity generation from nuclear energy uses the same basic process as electricity generation from fossil fuels: Steam turns a turbine that turns a generator that generates electricity. The difference is that a nuclear power plant uses a radioactive isotope, uranium-
Fission A nuclear reaction in which a neutron strikes a relatively large atomic nucleus, which then splits into two or more parts, releasing additional neutrons and energy in the form of heat.
Fission, depicted in FIGURE 36.1, is a nuclear reaction in which a neutron strikes a relatively large atomic nucleus, which then splits into two or more parts. This process releases additional neutrons and energy in the form of heat. The additional neutrons can, in turn, promote additional fission reactions, which leads to a chain reaction of nuclear fission that gives off an immense amount of heat energy. In a nuclear power plant, that heat energy is used to produce steam, just as in any other thermal power plant. However, 1 g of 235U contains 2 million to 3 million times the energy of 1 g of coal.
Uranium-
1 neutron + 235U → 142Ba + 91Kr + 3 neutrons in
motion (kinetic energy)
Many other radioactive daughter products are released as well. A properly designed nuclear reactor will harness the kinetic energy from the three neutrons in motion to produce a self-
Fuel rod A cylindrical tube that encloses nuclear fuel within a nuclear reactor.
FIGURE 36.2 shows how a nuclear reactor works. The containment structure encloses the nuclear fuel—
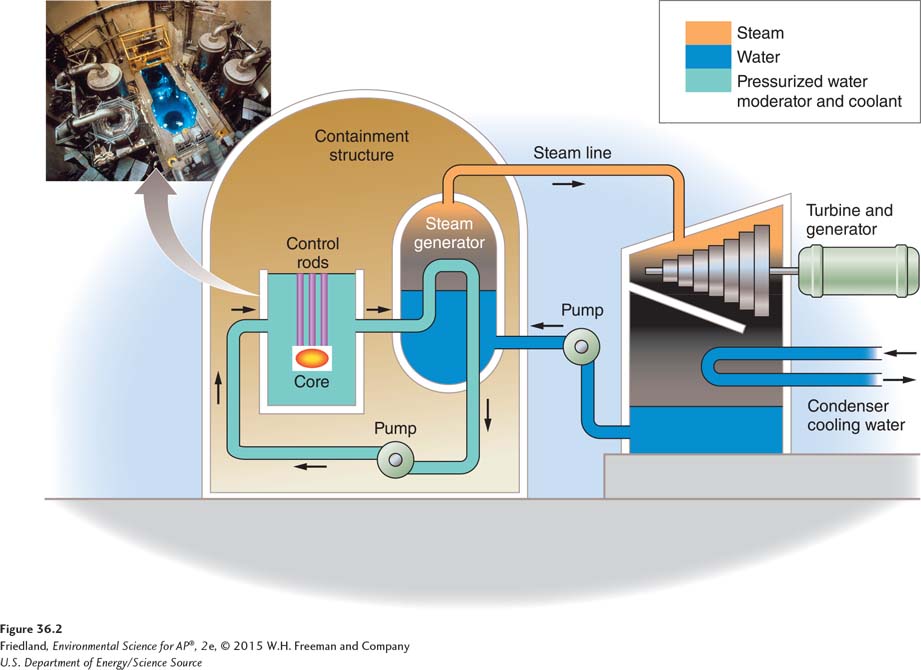
Heat from nuclear fission is used to heat water within the containment structure, which circulates in a loop. This loop passes close to another loop of water, and heat is transferred from one loop to the other. In the process, steam is produced, which turns a turbine, which turns a generator, just as in most other thermal power plants. The nuclear power plant shown in FIGURE 36.2 is a light-
Control rod A cylindrical device inserted between the fuel rods in a nuclear reactor to absorb excess neutrons and slow or stop the fission reaction.
A nuclear power plant is designed to harness heat energy from fission to make steam. But the plant must be able to slow the fission reaction to allow collisions to take place at the appropriate speed. To do this, nuclear reactors contain a moderator, such as water, to slow down the neutrons so that they can effectively trigger the next chain reaction. Because there is also a risk that the reaction will run out of control, nuclear reactors contain control rods, cylindrical devices that can be inserted between the fuel rods to absorb excess neutrons, thus slowing or stopping the fission reaction. This is done routinely during the operation of the plant because nuclear fuel rods left uncontrolled will quickly become too hot and melt—
Depending on the ore, it may take up to 900 kg (2,000 pounds) of uranium ore to produce 3 kg (6.6 pounds) of nuclear fuel. In order to obtain uranium, miners remove large amounts of the host rock, extract and concentrate the uranium, and leave the remaining material in slag piles. Australia, the western United States, and parts of Canada have large commercial uranium mining operations for nuclear fuel. As we saw in Chapter 8, the mining of any material requires fossil fuel energy and results in mine tailings. This is also true for uranium, although, as we have noted, a much smaller volume and mass of uranium is needed to generate a similar quantity of electricity than would be the case with coal.
Nuclear power plants rely on 235U as their fuel. However, most uranium ores contain as much as 99 percent 238U, another isotope of uranium that occurs with 235U but does not fission as easily. Therefore, when uranium ore is mined, it must be chemically enriched—
Nuclear energy has advantages and disadvantages
Nuclear power plants do not produce air pollution during their operation, so proponents of nuclear energy consider it “clean” energy. In countries with limited fossil fuel resources, nuclear energy is one way to achieve independence from imported oil. Nuclear energy generates 70 percent of electricity in France, and it is widely used in Lithuania, Germany, Spain, the United Kingdom, Japan, China, and South Korea, as well as in other countries.
As we saw in FIGURE 34.8, 20 percent of the electricity generated in the United States comes from nuclear energy. Early proponents of nuclear energy in the 1950s and 1960s claimed that it would be “too cheap to meter,” meaning that it would be so inexpensive that there would be no point in trying to figure out how much each customer used. However, construction of new nuclear power plants became more and more expensive in the United States, in part because public protests, legal battles, and other delays increased the cost of construction. Public protests arose because of concerns that a nuclear accident would release radioactivity into the surrounding air and water. Other concerns included uncertainty about appropriate locations for radioactive waste disposal and fear that radioactive waste could fall into the hands of individuals seeking to make a nuclear weapon. By the 1980s, it had become prohibitively expensive—
There are currently 104 nuclear power plants in the United States—
Nuclear Accidents
Two accidents contributed to the global protests against nuclear energy in the 1980s and 1990s. On March 28, 1979, at the Three Mile Island nuclear power plant in Pennsylvania, operators did not notice that a cooling water valve had been closed the previous day. This oversight led to a lack of cooling water around the reactor core, which overheated and suffered a partial meltdown. The reactor core was severely damaged, and a large part of the containment structure became highly radioactive with an unknown amount of radiation released to the outside environment. A few thousand schoolchildren and pregnant women were evacuated from the area surrounding the plant by order of the governor of Pennsylvania, although an estimated 200,000 other people chose to evacuate as well.
In the days following the accident, residents of the area experienced a great deal of anxiety and fear, especially as reports of a potentially explosive gas bubble in the containment structure were evaluated. Although there has been no documented increase in local health problems as a result of this accident, concerns remain and several investigators maintain that infant mortality rates and cancer rates increased in the following years. This nuclear reactor, one of two at the Three Mile Island nuclear facility, has not been used since the accident.
The Three Mile Island event, compounded by the coincidental release of the film The China Syndrome about safety violations and a near-
A much more serious accident occurred on April 26, 1986, at a nuclear power plant in Chernobyl, Ukraine. The accident occurred during a test of the plant when, in violation of safety regulations, operators deliberately disconnected emergency cooling systems and removed the control rods. With no control rods and no coolant, the nuclear reactions continued without control and the plant overheated. These “runaway” reactions led to an explosion and fire that damaged the plant beyond use. At the time of the accident, 31 plant workers and firefighters died from acute radiation exposure and burns, and many more died later of causes related to exposure.
After the accident, winds blew radiation from the plant across much of Europe, where it contaminated crops and milk from cows grazing on contaminated grass. More than a hundred thousand people were evacuated from the area around Chernobyl. Estimates of health effects vary widely, in part because of the paucity of information provided by the Soviet government, but a U.S. National Academy of Sciences panel estimated that 4,000 additional cancer deaths (over and above the average number of expected deaths) would occur over the next 50 years among people who lived near the plant or worked on the cleanup. There have been approximately 5,000 cases of thyroid cancer, most of them nonfatal, among children who were younger than 18 at the time of the accident and lived near the Chernobyl plant. Thyroid cancer may be caused by the absorption of radioactive iodine, one of the radioactive elements emitted during the accident.
An accident considered to be almost equivalent in severity to Chernobyl occurred in Japan on March 11, 2011. A magnitude 9.0 earthquake off the country’s coast generated a tsunami that was 15 m high. This caused flooding in northeastern Japan, where the Fukushima nuclear power plant is located. The resulting structural damage to the plant and interruption of the regional electrical supply led to fires, hydrogen gas explosions, and the release of radioactive gases from nuclear reactors within the containment structure. Ultimately, four out of a total of six reactors were damaged beyond repair, radioactive gases were released into the surrounding environment, and over 100,000 people were evacuated from their homes. Approximately 20,000 people, including 3 workers at the Fukushima plant, were killed by the earthquake and tsunami. However, at present, there have been no deaths attributed to the release of radioactivity from the reactors.
Radioactive Waste
Radioactive waste Nuclear fuel that can no longer produce enough heat to be useful in a power plant but continues to emit radioactivity.
When nuclear fuel is no longer useful in a power plant, yet continues to emit radioactivity, it is considered radioactive waste. Long after nuclear fuel can produce enough heat to be useful in a power plant, it continues to emit radioactivity. Because radioactivity can be extremely damaging to living organisms, radioactive materials—
The use of nuclear fuels produces three kinds of radioactive waste: high-
After a period of time, nuclear fuel rods become spent, meaning they are not sufficiently radioactive to generate electricity efficiently. As we discussed in Chapter 2, each radioactive isotope has a specific half-
Becquerel (Bq) Unit that measures the rate at which a sample of radioactive material decays; 1 Bq = decay of 1 atom or nucleus per second.
Curie A unit of measure for radiation; 1 curie = 37 billion decays per second.
Radiation can be measured with a variety of units. A becquerel (Bq) measures the rate at which a sample of radioactive material decays; 1 Bq is equal to the decay of one atom per second. A curie, another unit of measure for radiation, is 37 billion decays per second. If a material has a radioactivity level of 100 curies and has a half-
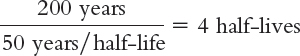
100 curies → 50 curies (one half-
You can gain more experience in working with these calculations in “Do the Math: Calculating Half-

Because spent fuel rods remain a threat to human health for 10 or more half-
Disposing of radioactive waste is a challenge. This waste cannot be incinerated, safely destroyed using chemicals, shot into space, dumped on the ocean floor, or buried in an ocean trench because all of these options involve the potential for large amounts of radioactivity to enter the oceans or atmosphere. Therefore, at present, the only solution is to store it safely somewhere on Earth indefinitely. The physical nature of the storage site must ensure that the waste will not leach into the groundwater or otherwise escape into the environment. It must be far from human habitation in case of any accidents and secure against terrorist attack. In addition, the waste has to be transported to the storage site in a way that minimizes the risk of accidents or theft by terrorists.
In 1978, the U.S. Department of Energy began examining a site at Yucca Mountain, Nevada, 145 km (90 miles) northwest of Las Vegas, as a possible long-
Nuclear energy raises a unique question of sustainability. It is a source of electricity that releases much less CO2 than coal, and none during the actual generation of electricity. But some argue that the use of a fuel that consistently generates large quantities of high-
Fusion Power
Nuclear fusion A reaction that occurs when lighter nuclei are forced together to produce heavier nuclei.
As we have seen, nuclear fission occurs when the nuclei of radioactive atoms are broken apart into smaller, lighter nuclei. Nuclear fusion, the reaction that powers the Sun and other stars, occurs when lighter nuclei are forced together to produce heavier nuclei. In the process, a great deal of heat is generated. The nuclear fusion reaction that is most promising for electricity generation is that of two hydrogen isotopes fusing together into a helium atom. As the reaction occurs, a small amount of mass is lost and an immense amount of energy is liberated.
Nuclear fusion seems to promise a seemingly unlimited source of energy that requires only hydrogen as an input and produces relatively small amounts of radioactive waste. Unfortunately, creating fusion on Earth requires a reactor that will heat material to temperatures 10 times those in the core of the Sun. These temperatures make containment extremely difficult. So far, the most promising techniques have involved suspending superhot material in a magnetic field, but the amount of energy required is greater than the energy output. Most experts believe that it will be several decades, or perhaps longer, before the promise of fusion power can be realized.
Summary of Nuclear Power and Comparison with Other Fuels
At present, our reliance on nuclear energy in the United States is subject to speculation and projections, but it is hard to know whether the positive aspects of nuclear energy will come to outweigh the risks of accidents and containment of radioactive waste. Indeed, in 2006, applications for new nuclear power plants in the United States were filed with the Nuclear Regulatory Commission for the first time in more than 2 decades. In 2012, licenses were approved for two nuclear power plants. It does appear that up to five new nuclear power plants may come online in the United States in the next decade.
TABLE 36.1 summarizes the major benefits and consequences of the conventional fuels we have discussed in this chapter.
