module 42 Heavy Metals and Other Chemicals
We have seen that some compounds such as nitrogen and phosphorus cause environmental problems by overfertilizing the water. Other inorganic compounds, including heavy metals (lead, arsenic, and mercury), acids, and synthetic compounds (pesticides, pharmaceuticals, and hormones), can directly harm humans and other organisms. In this module, we will examine how each of these chemicals enters water bodies and the effects that they can have on ecosystems and humans.
Learning Objectives
After reading this module you should be able to
explain the sources of heavy metals and their effect on organisms.
discuss the sources and effects of acid deposition and acid mine drainage.
explain how synthetic organic compounds can affect aquatic organisms.
Heavy metals are highly toxic to organisms
Heavy metals are a group of chemicals that can pose serious health threats to humans and other organisms. In this section, we will focus on three heavy metals that are of particular concern: lead, arsenic, and mercury.
Lead
Lead is rarely found in natural sources of drinking water, but it contaminates water that passes through lead-
Arsenic
Arsenic is a compound that occurs naturally in Earth’s crust and can dissolve into groundwater. As a result, naturally occurring arsenic in rocks can lead to high concentrations of arsenic in groundwater and drinking water. Human activity also contributes to higher arsenic concentrations in groundwater. For example, mining breaks up rocks deep underground, and industrial uses of arsenic for items such as wood preservatives can add to the amount of arsenic found in drinking water. Fortunately, arsenic can be removed from water via fine membrane filtration, distillation, and reverse osmosis (see FIGURE 27.6 on page 306).
FIGURE 42.1 indicates where high concentrations of arsenic have been found in well water throughout the United States. As you can see, concentrations are highest in the Midwest and West. Arsenic in drinking water is associated with cancers of the skin, lungs, kidneys, and bladder. These illnesses can take 10 years or more after exposure to develop. Even very low concentrations of arsenic, such as those found in many wells around the world and measured in parts per billion (ppb), can cause severe health problems. In fact, though cancers can develop with less than 50 ppb of arsenic in drinking water, 50 ppb was set as the upper “safe” limit for U.S. drinking water from 1942 to 1999. In 1999, the EPA lowered the upper limit to 10 ppb, a compromise reached after much debate between environmental groups pushing for 5 ppb and water, mining, and wood preservative industries arguing that even the 10 ppb limit would be too expensive to implement. In 2001, the EPA delayed the implementation of the 10 ppb standard until more data could be evaluated. Later that year, the U.S. National Academy of Sciences recommended a standard of 5 ppb and the EPA decided to implement a standard of 10 ppb. As a result, after substantial investment required to improve many water treatment facilities, people in the United States will now have lower amounts of cancer-
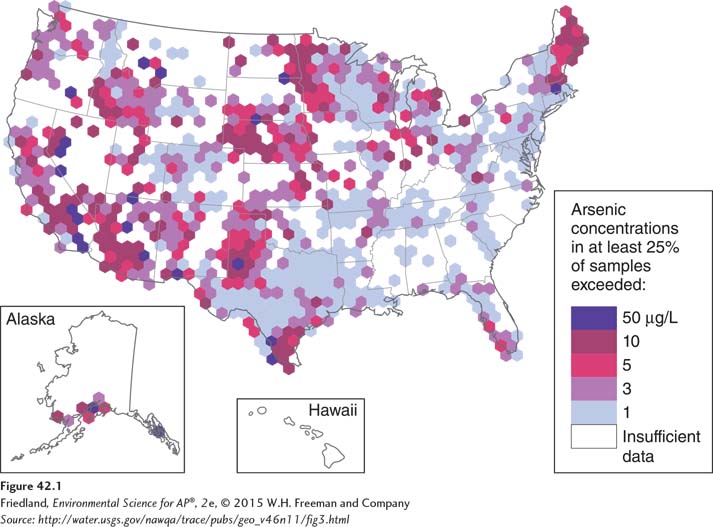
The problem of arsenic in drinking water is a worldwide issue. During the 1980s and 1990s, for example, water engineers in regions of Bangladesh and eastern India drilled millions of very deep wells in an attempt to find sources of groundwater that were not contaminated by local pollution. While this had the desired effect of obtaining less-
Mercury
Mercury is another naturally occurring heavy metal found in increased concentrations in water as a result of human activities. FIGURE 42.2 shows mercury releases from different regions of the world as the result of activities such as burning coal (see Chapter 12). Among regions of the world, 6 percent of human-
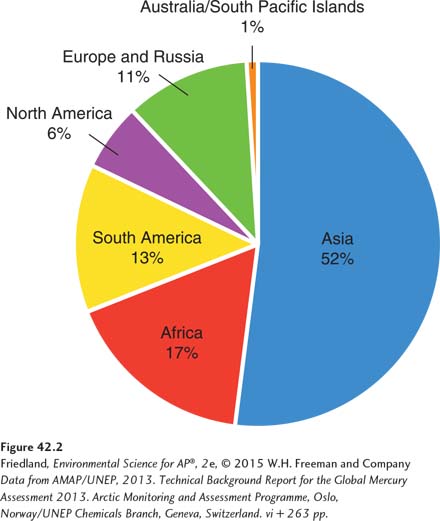
Approximately two-
Petroleum exploration is a source of both mercury and lead pollution. Each petroleum well produces roughly 681,374 L (180,000 gallons) of contaminated wastewater and mud over its lifetime. This water is usually dumped at the drilling site and, depending on the soils and topography, can either run into nearby waterways or infiltrate the soil and contaminate the underlying groundwater.
The mercury emitted by these activities eventually finds its way into water. Inorganic mercury (Hg) is not particularly harmful, but its release into the environment can be hazardous because of a chemical transformation it undergoes. In wetlands and lakes, bacteria convert inorganic mercury into methylmercury, which is highly toxic to humans. Methylmercury damages the central nervous system, particularly in young children and in the developing embryos of pregnant women. The result impairs coordination and the senses of touch, taste, and sight.
Human exposure to methylmercury occurs mostly through eating fish and shellfish. Methylmercury can move up the food chain in aquatic ecosystems, which results in the top consumers containing the highest concentrations of mercury in their bodies. Given that oceans are contaminated with mercury and that tuna are top predators, it is not surprising that these fish contain high concentrations of mercury. In 2008, a reporter for the New York Times purchased tuna sushi from 20 locations in New York City and, after analysis by a private laboratory, found that, at most restaurants, a diet of six pieces of sushi per week would exceed the EPA standard for human consumption of mercury. In 2009, the EPA reported that the concentration of mercury in the tuna of the North Pacific Ocean had increased by 30 percent since 1990. If China’s plans to build more electric-
What can be done about mercury pollution? In 2013 the United States announced a new agreement with more than 140 other countries to reduce global mercury pollution. In a step toward this goal, the EPA has proposed that cement-
Acid deposition and acid mine drainage affect terrestrial and aquatic ecosystems
Acid deposition Acids deposited on Earth as rain and snow or as gases and particles that attach to the surfaces of plants, soil, and water.
About 40 years ago, people throughout the northeastern United States, northern Europe, China, and Russia began to notice that the forests, soils, lakes, and streams were becoming more and more acidic. As a consequence, some trees were killed and some bodies of water became too acidic to sustain fish. After much debate, it became clear that the source of the lower pH of the water was the presence of very tall smokestacks of industrial plants that were burning coal and releasing sulfur dioxide and nitrogen dioxide into the air. These tall smokestacks kept the emissions away from local residents, but sent the chemicals into the atmosphere where they were converted into sulfuric acid and nitric acid that returned to Earth hundreds of kilometers away. Acids deposited on Earth as rain and snow or as gases and particles that attach into the surfaces of plants, soil, and water are known as acid deposition. As described in Chapter 8, wet-
To combat the problem of acids being released into the atmosphere, many coal-
Low pH in water bodies also occurs when very acidic water comes from below ground. This problem begins with the development of underground mines that, once abandoned, flood with groundwater. The combination of water and air allows pyrite, a type of rock, to break down and produce iron and hydrogen ions. The increase in hydrogen ions produces acidic water with a low pH. Water in the mine containing these ions can find its way up to the surface in the form of springs that feed into streams. As we discussed in Chapter 8, a similar effect occurs during mountaintop mining operations in which the tops of mountains are removed and the soil is dumped into stream valleys. The streams that are fed by springs from these mines are infiltrated with water that can have a pH close to zero. Low-

Unfortunately, many mining companies responsible for making streams uninhabitable for fish and other organisms are no longer in business. As a result, they are often not held accountable for the environmental damage they caused. This presents a major challenge for state and local governments that bear the cost of dealing with the problem of acidic water from mines and the toxic metals that are produced. Researchers are currently investigating a number of strategies to counteract the acidity of these streams. One possible approach is to pass stream water through a limestone treatment facility that raises the pH of the water and removes toxic metals to levels tolerable to stream organisms.
Synthetic organic compounds are human-produced chemicals
Synthetic, or human-
Pesticides and Inert Ingredients
Pesticides serve an important role in helping to control pest organisms that pose a threat to crop production and human health (FIGURE 42.4). Although natural pesticides such as arsenic have been used for centuries, the first generation of synthetic pesticides was developed during World War II. As we discussed in Chapter 11, these chemicals proved to be very effective in killing a variety of undesired plants (herbicides), fungi (fungicides), and insects (insecticides). In the decades that have followed, however, environmental scientists have identified a number of concerns about the unintended effects of pesticides.

Most pesticides do not target particular species of organisms, but generally kill a wide variety of related organisms. For example, an insecticide that is sprayed to kill mosquitoes is typically lethal to many other species of invertebrates, including insects that might be desirable as predators of the mosquito. Some pesticides are lethal to unrelated species. For example, researchers have recently discovered that the insecticide endosulfan, a chemical designed to kill insects, is highly lethal to amphibians even at very low concentrations. Even a pesticide that is not directly lethal to a species can indirectly affect organisms by altering the species composition of the community.
Synthetic pesticides are generally designed specifically to target particular aspects of a pest’s physiology. However, they can also alter physiological functions of other species. For example, most insecticides target the nervous system of a particular pest, yet they can have unintended impacts on other pests as well as on many nonpest species. The insecticide DDT (dichlorodiphenyltrichloroethane) is a good example. While DDT was designed to alter nerve transmissions in insects, the chemical can move up an aquatic food chain all the way to eagles that consume fish. Eagles that consumed DDT-
Another concern about pesticides is the effect of inert ingredients added to commercial formulations. Inert ingredients are additives that make a pesticide more effective—
Pharmaceuticals and Hormones
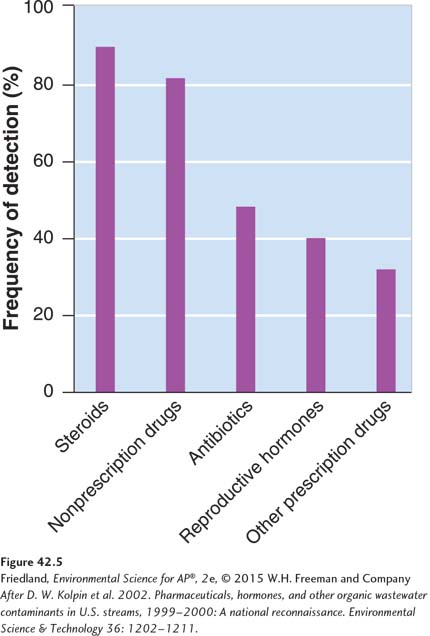
While most people know that pesticides are commonly found in the environment, they often are surprised to learn that pharmaceutical drugs are also common. For example, the U.S. Geological Survey tested 139 streams across the United States for a variety of chemical contaminants. FIGURE 42.5 shows data for the frequency of detection. Among the different types of chemicals that were present at detectable levels, approximately 50 percent of all streams contained antibiotics and reproductive hormones, 80 percent contained nonprescription drugs, and 90 percent contained steroids. In most cases the concentrations of these chemicals are quite low and currently are not thought to pose a risk to environmental or human health. Some chemicals such as hormones, however, operate at very low concentrations inside the tissues of organisms and we have a poor understanding of their effects. As noted in our discussion of the Chesapeake Bay, it is now clear that low concentrations of pharmaceutical drugs that mimic estrogen are connected to male fish growing eggs in their testes. The extent of hormone effects on humans and wildlife around the world is currently unknown but environmental scientists are showing increased attention to these effects. We will talk more about the ways that chemicals can alter the endocrine systems of animals in Chapter 17.
Military Compounds
Perchlorates A group of harmful chemicals used for rocket fuel.
Perchlorates, a group of harmful chemicals used for rocket fuel, sometimes contaminate the soil in regions of the world where military rockets are manufactured, tested, or dismantled. Perchlorates come in many forms. The U.S. space shuttle, for example, used a booster rocket that contained 70 percent ammonium perchlorate. Perchlorates easily leach from contaminated soil into the groundwater, where they can persist for many years. Human exposure to perchlorates comes primarily through consumption of contaminated food and water. In the human body, perchlorates can affect the thyroid gland and reduce the production of hormones necessary for proper functioning of the human body.
Industrial Compounds
Industrial compounds are chemicals used in manufacturing. Unfortunately, it used to be common for manufacturers in the United States to dispose of industrial compounds directly into bodies of water. One of the most widely publicized consequences of this practice occurred in the Cuyahoga River of Ohio. For more than 100 years, industries along the river dumped industrial wastes that formed a slick of pollution along the surface, killing virtually all animal life. The problem became so bad that the river actually caught fire and burned several times over the decades (FIGURE 42.6). The fire on the river in 1969 garnered national attention and led to a movement to clean up America’s waterways. Today, the Cuyahoga River and most other rivers in the United States are much cleaner because of legislation that substantially reduced the amount of industrial and other waste that can be legally dumped into waterways.

Polychlorinated biphenyls (PCBs) A group of industrial compounds used to manufacture plastics and insulate electrical transformers, and responsible for many environmental problems.
Polychlorinated biphenyls (PCBs) are a group of industrial compounds that were once used to manufacture plastics and insulate electrical transformers. PCBs have caused many environmental problems. Ingested PCBs are lethal and carcinogenic, or cancer-
While PCBs have long been a concern, there is a growing uneasiness over compounds known as PBDEs (polybrominated diphenyl ethers). PBDEs are most commonly known as flame retardants added to a wide variety of items that include construction materials, furniture, electrical components, and clothing. They make buildings and their contents considerably less flammable than they would be otherwise. Since the 1990s, however, scientists have been detecting PBDEs in some unexpected places, including fish, aquatic birds, and human breast milk. Exposure to some types of PBDEs can lead to brain damage, especially in children. As a result, the European Union and several states, including Washington and California, have banned the manufacture of several types of PBDEs.