module 46 Major Air Pollutants and Their Sources
Air pollution The introduction of chemicals, particulate matter, or microorganisms into the atmosphere at concentrations high enough to harm plants, animals, and materials such as buildings, or to alter ecosystems.
Air pollution is defined as the introduction of chemicals, particulate matter, or microorganisms into the atmosphere at concentrations high enough to harm plants, animals, and materials such as buildings, or to alter ecosystems. Generally, the term air pollution refers to pollution in the troposphere, the first 16 km (10 miles) of the atmosphere above the surface of Earth. Tropospheric pollution is also sometimes called ground-
In this module, we will examine the major air pollutants that occur in the troposphere and where they come from.
Learning Objectives
After reading this module, you should be able to
identify and describe the major air pollutants.
describe the sources of air pollution.
Air pollution is a global system
Since one of the major repositories for air pollutants is the atmosphere, which envelops the entire globe, we must think of the air pollution system as a global system. In fact, evidence appears to link air pollution across long distances. For example, in recent years, air pollution in Asia has been responsible for acidic rainfall on the West Coast of the United States (FIGURE 46.1).

The air pollution system has many inputs, which are the sources of pollution. It also has many outputs, which are components of the atmosphere and biosphere that remove air pollutants. It is difficult to conceptualize this system because the inputs do not originate from just one location. Air pollution inputs can come from automobiles on the ground, airplanes in the sky, or vegetation (tree leaves) 100 feet in the air. Similarly, air pollution can be removed or altered by vegetation, soil, and components of the atmosphere such as clouds, particles, or gases.
As a starting point for understanding the global air pollution system, we will identify the major pollutants and determine where they come from.
Classifying Pollutants
Even though air pollution has been with us for millennia, both the specific definition of pollution and the classification of a substance as a pollutant have evolved. The atmosphere is a public resource—
Although carbon dioxide was not included among the major air pollutants identified in the 1970s, today it is widely accepted that carbon dioxide is altering ecosystems in a substantial way. In 2007, the U.S. Supreme Court ruled that carbon dioxide should be considered an air pollutant under the Clean Air Act, and in 2012, a federal appeals court agreed that the EPA is required to impose limits on harmful greenhouse gas emissions, including carbon dioxide. In addition, volatile organic compounds and mercury, though not officially listed in the Clean Air Act, are commonly measured air pollutants that have the potential to be harmful.
The sources and effects of the major air pollutants, including the six criteria air pollutants, are summarized in TABLE 46.1. Let’s take a closer look at each of these major air pollutants.
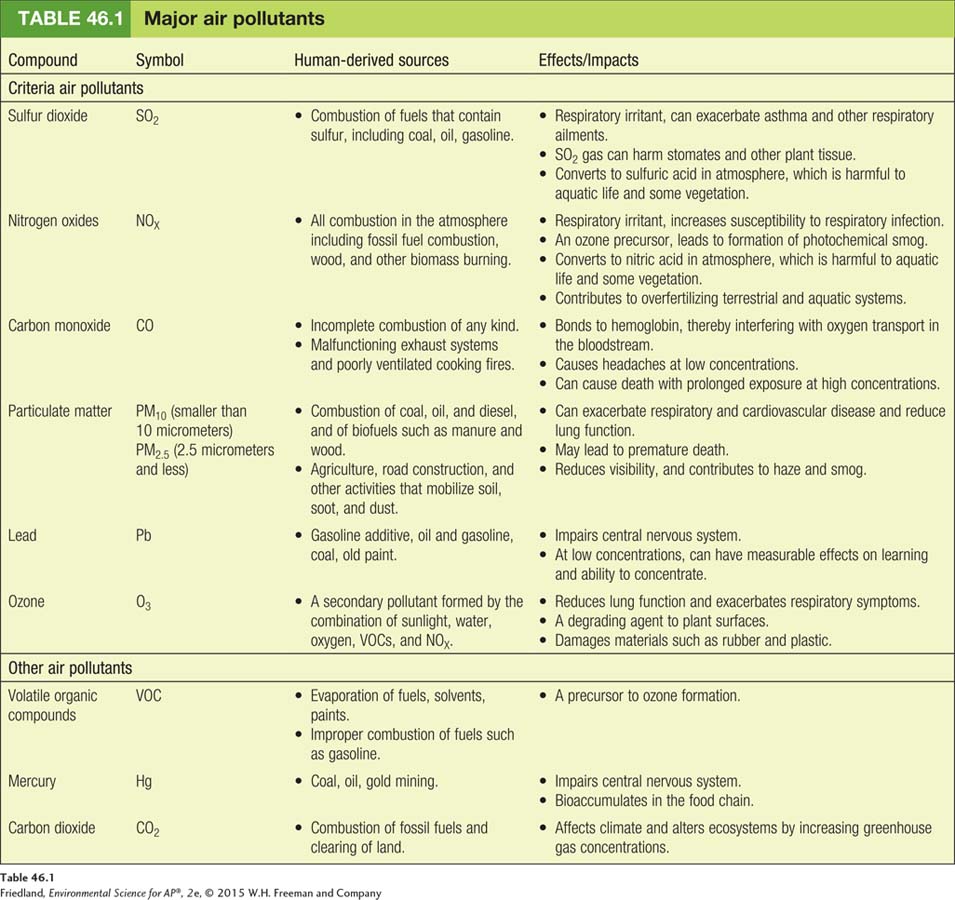
Sulfur Dioxide
Sulfur dioxide (SO2) is a corrosive gas that comes primarily from combustion of fuels such as coal and oil. It is a respiratory irritant and can adversely affect plant tissue as well. Because all plants and animals contain sulfur in varying amounts, the fossil fuels derived from their remains contain sulfur. When these fuels are combusted, the sulfur combines with oxygen to form sulfur dioxide. Sulfur dioxide is also released in large quantities during volcanic eruptions and can be released, though in much smaller quantities, during forest fires.
Nitrogen Oxides
Nitrogen oxides are generically designated NOX, with the X indicating that there may be either one or two oxygen atoms per nitrogen atom: nitrogen oxide (NO), a colorless, odorless gas; and nitrogen dioxide (NO2), a pungent, reddish-
Carbon Oxides
Carbon monoxide (CO) is a colorless, odorless gas that is formed during incomplete combustion of most matter, and therefore is a common emission in vehicle exhaust and most other combustion processes. Carbon monoxide can be a significant component of air pollution in urban areas. It also can be a dangerous indoor air pollutant when exhaust systems on natural gas heaters malfunction. Carbon monoxide is a particular problem in developing countries, where people may cook with manure, charcoal, or kerosene within poorly ventilated structures.
Carbon dioxide (CO2) is a colorless, odorless gas that is formed during the complete combustion of most matter, including fossil fuels and biomass. It is absorbed by plants during photosynthesis and is released during respiration. In general, the complete combustion of matter that produces carbon dioxide is more desirable than the incomplete combustion that produces carbon monoxide and other pollutants. However, burning fossil fuels has contributed additional carbon dioxide to the atmosphere and led to its becoming a major pollutant. It recently exceeded a concentration of 400 parts per million in the atmosphere and appears to be steadily increasing each year. This topic will be covered in more detail in Chapter 19 where we discuss the issue of climate change.
Particulate Matter
Particulate matter (PM) Solid or liquid particles suspended in air. Also known as Particulates; Particles.
Particulate matter (PM), also called particulates or particles, is solid or liquid particles suspended in air. FIGURE 46.2 shows the sources of particulate matter and its effects. Particulate matter comes from the combustion of wood, animal manure and other biofuels, coal, oil, and gasoline. It is most commonly known as a class of pollutants released from the combustion of fuels such as coal and oil. Diesel-
Particulate matter ranges in size from 0.01 micrometer (µm) to 100 µm (1 micrometer = 0.000001 m). For comparison, a human hair has a diameter of roughly 50 to 100 µm. Particulate matter larger than 10 µm is usually filtered out by the nose and throat; particulate matter of this size is not regulated by the EPA. Particles smaller than 10 µm are called Particulate Matter-
Particulate matter also scatters and absorbs sunlight. If the atmospheric concentration of particulate matter is high enough, as it would be immediately following a large forest fire or a volcanic eruption, incoming solar radiation in the region will be reduced enough to affect photosynthesis. This happened in 1816, a year after a large volcanic eruption in Java released more than 150 million metric tons of particles that slowly spread around the globe. That year is commonly referred to as the year without a summer.
Haze Reduced visibility.
Reduced visibility, also known as haze, occurs primarily when particulate matter from air pollution scatters light. But as we will see in the next section, ozone and photochemical oxidants can also play an important indirect role in the formation of haze.
Photochemical Oxidants
Photochemical oxidant A class of air pollutants formed as a result of sunlight acting on compounds such as nitrogen oxides.
Ozone (O3) A secondary pollutant made up of three oxygen atoms bound together.
Oxides are reactive compounds that remove electrons from other substances. Photochemical oxidants are a class of air pollutants formed as a result of sunlight (photo) acting on chemical compounds such as nitrogen oxides and sulfur dioxide. There are many photochemical oxidants, and they are generally harmful to plant tissue, human respiratory tissue, and construction materials. However, environmental scientists frequently focus on ozone. Ozone (O3) is a secondary pollutant made up of three oxygen atoms bound together. It is harmful to both plants and animals and impairs respiratory function.
Smog A type of air pollution that is a mixture of oxidants and particulate matter.
Photochemical smog Smog that is dominated by oxidants such as ozone. Also known as Los Angeles–
Sulfurous smog Smog dominated by sulfur dioxide and sulfate compounds. Also known as London-
In the presence of sulfur and nitrogen oxides, photochemical oxidants can enhance the formation of certain particulate matter, which contributes to scattering light. The resulting mixture is called smog, a mixture of oxidants and particulate matter. The word is derived by combining smoke and fog. Smog is partly responsible for the hazy view and reduced sunlight observed in many cities. Smog can be divided into two categories. Photochemical smog is dominated by oxidants such as ozone and is sometimes called Los Angeles–
Atmospheric brown cloud is a relatively new descriptive term that has been given to the combination of particulate matter and ozone. Derived primarily from combustion of fossil fuel and burning biomass, atmospheric brown clouds have been observed in cities and throughout entire regions, especially in Asia. The brownish tint that characterizes these clouds of pollution is typically caused by the presence of black or brown light absorbing carbon particles and/or nitrogen dioxide.
In addition to human health problems, particulate matter and photochemical oxidants also cause economic harm, since poor visibility in popular vacation destinations can reduce tourism revenues for recreation areas, such as lower incomes for hotels and restaurants in these areas. We will examine photochemical smog in greater detail in the next module.
Lead and Other Metals
Lead (Pb) is a trace metal that occurs naturally in rocks and soils. It is present in small concentrations in fuels including oil and coal. Lead compounds were added to gasoline for many years to improve vehicle performance. During that time, lead compounds released into the air traveled with the prevailing winds and were deposited on the ground by rain or snow. They became pervasive around the globe, including in polar regions far from combustion sources.
Lead was phased out as a gasoline additive in the United States between 1975 and 1996, and since then its concentration in the air has dropped considerably. A campaign to phase out lead use in gasoline globally is still underway. Another persistent source of lead is lead-
Mercury (Hg), another trace metal, is also found in coal and oil and, like lead, is toxic to the central nervous system of humans and other organisms. The EPA regulates mercury through its hazardous air pollutants program. As a result of the release of mercury into the air, primarily from the combustion of fossil fuels, especially coal, the concentrations of mercury in both air and water have increased dramatically in recent years. As we will see in Chapter 17, mercury concentrations in some fish have also increased. People who eat these fish increase their own mercury concentrations—
Volatile Organic Compounds
Volatile organic compound (VOC) An organic compound that evaporates at typical atmospheric temperatures.
Organic compounds that evaporate at typical atmospheric temperatures are called volatile organic compounds (VOCs). Many VOCs are hydrocarbons—
Primary and Secondary Pollutants
When trying to understand pollution, and when attempting to reduce pollution emissions, it is important to understand if a particular pollutant is coming directly from an emission source such as a smokestack or tailpipe, or if it has undergone transformations after emission. Thus, pollutants in the air can be categorized as primary or secondary.
Primary Pollutants
Primary pollutant A polluting compound that comes directly out of a smokestack, exhaust pipe, or natural emission source.
Primary pollutants are polluting compounds that come directly out of a smokestack, exhaust pipe, or natural emission source. As you can see in FIGURE 46.3, they include CO, CO2, SO2, NOX, and most suspended particulate matter. Many VOCs are also primary pollutants. For example, as gasoline is burned in a car, it volatilizes from a liquid to a vapor, some of which is emitted from the exhaust pipe in an uncombusted form. The effect is more pronounced if the car is not operating efficiently. The resulting VOC becomes a primary air pollutant.
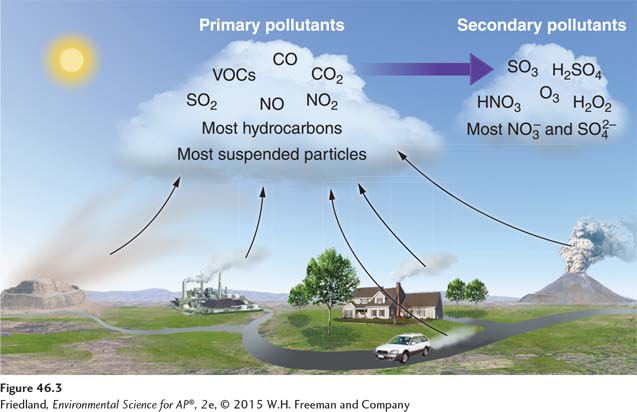
Secondary Pollutants
Secondary pollutant A primary pollutant that has undergone transformation in the presence of sunlight, water, oxygen, or other compounds.
Secondary pollutants are primary pollutants that have undergone transformation in the presence of sunlight, water, oxygen, or other compounds. Because solar radiation provides energy for many of these transformations, and because water is usually involved, the conversion to secondary pollutants occurs more rapidly during the day and in wet environments.
Ozone is an example of a secondary pollutant. Ozone is formed in the atmosphere as a result of the emission of the primary air pollutants NOX and VOCs in the presence of sunlight. The main components of acid deposition—
When trying to control secondary pollutants, it is necessary to consider the primary pollutants that create them, as well as factors that may lead to the breakdown or reduction in the secondary pollutants themselves. For example, when municipalities such as Chattanooga try to reduce ozone concentrations in the air, as described at the beginning of this chapter, they focus on reducing the compounds that lead to ozone formation—
Environmental scientists and concerned citizens direct much of their attention toward air pollution that comes from human activity. But human activity is not the only source of air pollution because processes in nature also cause air pollution. In this section we will examine both natural and human sources of air pollution.
Natural Emissions
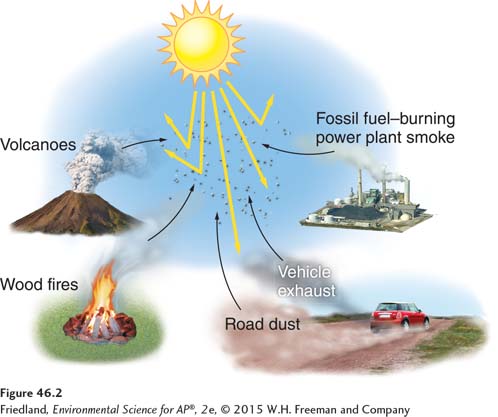
Volcanoes, lightning, forest fires, and plants both living and dead all release compounds that can be classified as pollutants. Volcanoes release sulfur dioxide, particulate matter, carbon monoxide, and nitrogen oxides. Lightning strikes create nitrogen oxides from atmospheric nitrogen. Forest fires release particulate matter, nitrogen oxides, and carbon monoxide (FIGURE 46.4a). Living plants release a variety of VOCs, including ethylene and terpenes. The fragrant smell from conifer trees such as pine and fir and the smell from citrus fruits are mostly from terpenes; though we enjoy their fragrance, they can be precursors to photochemical smog. Long before anthropogenic pollution was common, the natural VOCs from plants gave rise to smog and photochemical oxidant pollution—

The effects of these various compounds, especially when major natural events occur, depend in part on natural conditions such as wind direction. In May 1980, the prevailing westerly winds carried volcanic emissions from Mount St. Helens in Washington State and distributed particulate matter and sulfur oxides from the volcano across the United States. The rainfall pH was noticeably lower in the eastern United States that summer.
Anthropogenic Emissions
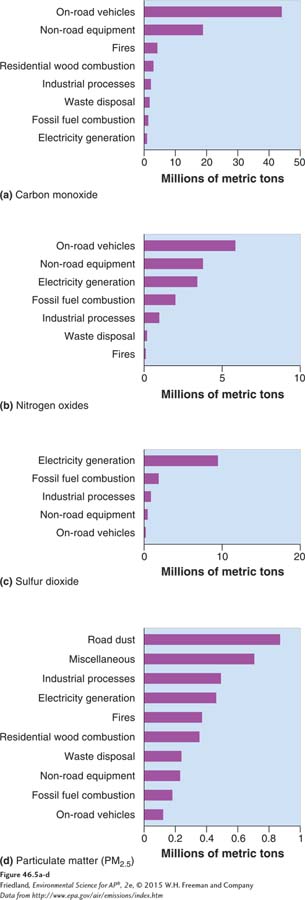
In contrast to natural emissions, emissions from human activity are monitored, regulated, and in many cases controlled. The EPA reports periodically on the emission sources of the criteria air pollutants for the entire United States, listing pollution sources in a variety of categories, such as on-
The Clean Air Act and its various amendments require that the EPA establish standards to control pollutants that are harmful to “human health and welfare.” The term human health means the health of the human population and includes the elderly, children, and sensitive populations such as those with asthma. The term welfare refers to visibility, the status of crops, natural vegetation, animals, ecosystems, and buildings. Through the National Ambient Air Quality Standards (NAAQS), the EPA periodically specifies concentration limits for each air pollutant. For each pollutant the NAAQS note a concentration that should not be exceeded over a specified time period. Our discussion of air pollution in Chattanooga at the beginning of this chapter partially described the standard for ozone: For each locality in the United States, the average ozone concentration for any 8-
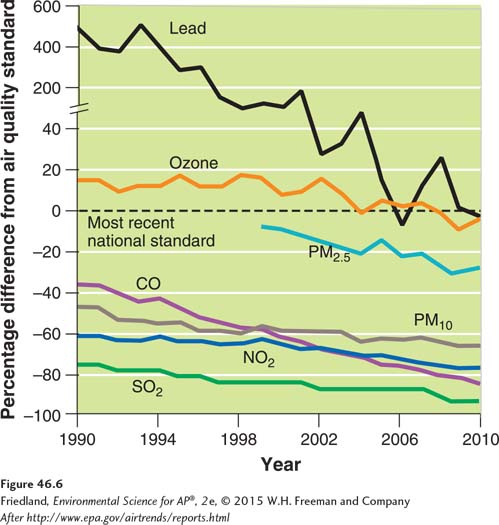
Each year, the EPA issues a report that shows the national level of the six criteria air pollutants relative to the published standards. FIGURE 46.6 shows that all criteria air pollutants have decreased in the United States over the last 2 decades. Only ozone and particulate matter concentrations exceed NAAQS on a regular basis. The cases where particulate matter exceeds national standards are not evident from FIGURE 46.6. Lead has decreased most significantly because it is no longer added to gasoline.
The situation is less positive in other parts of the world. Large areas in Germany, Poland, and the Czech Republic contain a great deal of “brown” coal or lignite that provides fuel for nearby coal-