module 47 Photochemical Smog and Acid Rain
The air quality in the United States has greatly improved in recent decades. However, some cities and even regions of the United States continue to experience intermittent air pollution, often related to smog formation. In this module, we will examine photochemical smog, which is a problem in the United States and elsewhere in the world, and acid rain, which is no longer a problem in the United States but has become a problem in Asia.
Learning Objectives
After reading this module, you should be able to
explain how photochemical smog forms and why it is still a problem in the United States.
describe how acid deposition forms and why it has improved in the United States and become worse elsewhere.
Photochemical smog remains an environmental problem in the United States
A recent headline read, “EPA Says Half of the United States Is Breathing Excessive Levels of Smog.” You might think this was from a newspaper in the 1970s, before the Clean Air Act was fully in effect. But in December 2013, the EPA reported that 46 regions within the United States did not comply with the maximum allowable ozone concentration in the air of 0.075 parts of ozone per million parts of air over an 8-
The Chemistry of Ozone and Photochemical Smog Formation
As we mentioned earlier, the term smog was originally used to describe the combination of smoke, fog, and sometimes sulfur dioxide that used to occur in cities that burned a lot of coal. Today, Los Angeles–
FIGURE 47.1 shows a portion of the chemical process that creates photochemical smog. The first part of the process, shown in FIGURE 47.1a, takes place during the day, in the presence of sunlight. When an abundance of nitrogen oxides are present in the atmosphere, with very few VOCs present, nitrogen dioxide (NO2) splits to form nitrogen oxide (NO) and a free oxygen atom (O). In the presence of energy inputs from sunlight, this free oxygen atom combines with diatomic oxygen (O2) to form ozone (O3). With abundant nitrogen dioxide and abundant sunlight, ozone can accumulate in the atmosphere.
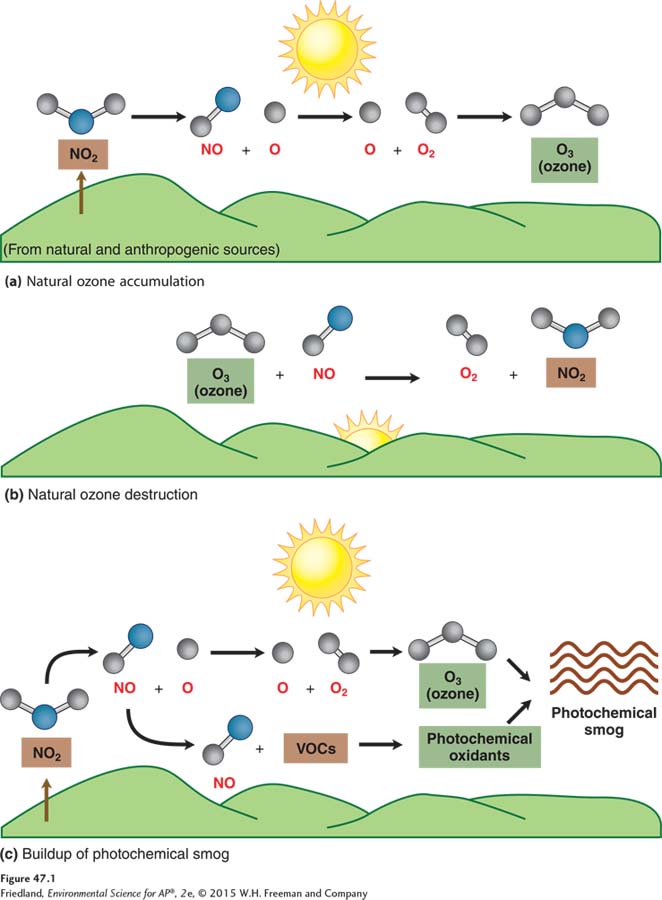
FIGURE 47.1b shows that a few hours later, when sunlight intensity decreases and with nitrogen oxide still present in the atmosphere, the ozone combines with nitrogen oxide (NO), and re-
Volatile organic compounds come from human activity such as spilling of gasoline on pavement and from natural sources such as forests. When volatile organic compounds are absent or in small supply, the cycle of ozone formation and destruction generally takes place on a daily basis and relatively small amounts of photochemical smog form.
As shown in FIGURE 47.1c, a different scenario occurs when VOCs are present. The first part is the same: Sunlight causes nitrogen dioxide to break apart into nitrogen oxide and a free oxygen atom. The free oxygen atom combines with diatomic oxygen to form ozone. However, because VOCs have combined with nitrogen oxide in a strong bond, nitrogen oxide is no longer available to combine with ozone. Since the nitrogen oxide is not available to break down ozone by recombining with it, a larger amount of ozone accumulates. This explains, in part, the daytime accumulation of ozone in urban areas with an abundance of both VOCs and nitrogen dioxide.
Although smog is associated with urban areas, it is not limited to such areas. Trees and shrubs in rural areas produce VOCs that can contribute to the formation of photochemical smog, as do forest fires that begin naturally.
Atmospheric temperature influences the formation of smog in several important ways. Emissions of VOCs from vegetation such as trees, as well as from evaporation of volatile liquids like gasoline, increase as the temperature increases. NOX emissions from electric utilities are also greater with air-
Thermal Inversions
Thermal inversion A situation in which a relatively warm layer of air at mid-
Inversion layer The layer of warm air that traps emissions in a thermal inversion.
Temperature also influences air pollution conditions in more complex ways. Normally, temperature decreases as altitude increases. As shown in FIGURE 47.2a, the warmest air is closest to Earth. This warm air, which is less dense than the colder air above it, can easily rise, dispersing pollutants into the upper atmosphere. This allows pollutants from the surface to be reduced or diluted by all of the atmosphere above. However, during a thermal inversion—shown in FIGURE 47.2b—a relatively warm layer of air at mid-
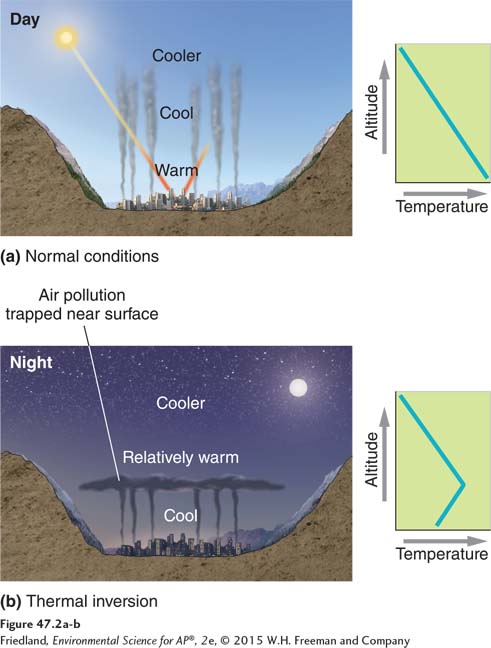
Thermal inversions can also lead to other forms of pollution. A striking example occurred in spring 1998 in the northern Chinese city of Tianjin. A cold spell that occurred after the city had shut off its central heating system for the season led many households to use individual coal-
Acid deposition has improved in the United States
All rain is naturally somewhat acidic; the reaction between water and atmospheric carbon dioxide lowers the pH of precipitation from neutral 7.0 to 5.6 (see FIGURE 4.7 on page 39). In Chapter 14 we described acid deposition, which refers to deposition with a pH lower than 5.6. Acid deposition is largely the result of human activity, although natural processes, such as volcanoes, may also contribute to its formation. In this section we will look at how acid deposition is formed, how it travels, and its effects.
How Acid Deposition Forms and Travels
FIGURE 47.3 shows how acid deposition forms. Nitrogen oxides (NO and NO2) and sulfur dioxide (SO2) are released into the atmosphere by natural and anthropogenic combustion processes. Through a series of reactions with atmospheric oxygen and water, these primary pollutants are transformed into the secondary pollutants nitric acid (HNO3) and sulfuric acid (H2SO4). These latter compounds break down further, producing nitrate, sulfate—
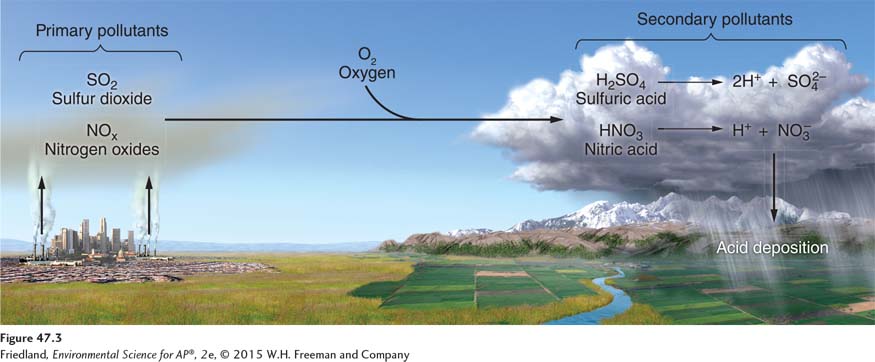
Acid deposition has been reduced in the United States as a result of lower sulfur dioxide and nitrogen oxide emissions, as shown in FIGURE 46.6. Much of this improvement is a result of the Clean Air Act Amendments that were passed in 1990 and implemented in 1990 and 1995.
Studies have documented regional acid deposition in West Africa, South America, Japan, China, and many areas in eastern and central Europe. Acid deposition crosses the border between the United States and Canada and is carried from England, Germany, and the Netherlands to Scandinavia. Because of this mobility, the precursors to acid deposition emitted in one region may have a significant impact on another region or another country. For example, over the years, there have been legislative and legal attempts to restrict emissions from coal-
Effects of Acid Deposition
As we saw in Chapter 14, acid deposition in the United States increased substantially from the 1940s through the 1990s due to human activity. It had a variety of effects on materials, on agricultural lands, and on both aquatic and terrestrial natural habitats. Newspaper headlines in the United States and Europe in the 1980s contained frequent reports about adverse effects of acid deposition on forests, lakes, and streams.
Effects of acid deposition may be direct, such as a decrease in the pH of lake water, or indirect. It is often difficult to determine whether an effect is direct or indirect, making remediation challenging. The greatest effects of acid deposition have been on aquatic ecosystems. Lower pH of lakes and streams in areas of northeastern North America, Scandinavia, and the United Kingdom has caused decreased species diversity of aquatic organisms. As we saw in Chapter 6, many species are able to survive and reproduce only within a narrow range of environmental conditions. Many amphibians, for instance, will survive when the pH of a lake is 6.5, but when the lake acidifies to pH 6.0 or 5.5, the same organism will begin to have developmental or reproductive problems. In water below pH 5.0, most salamander species cannot survive.

Lower pH can also lead to mobilization of metals, an indirect effect. When this happens, metals bound in organic or inorganic compounds in soils and sediments are released into surface water. Because metals such as aluminum and mercury can impair the physiological functioning of aquatic organisms, exposure can lead to species loss. Decreased pH can also affect the food sources of aquatic organisms, creating indirect effects at several trophic levels. On land, at least one species of tree, the red spruce (Picea rubens), at high elevations of the northeastern United States was shown to have been harmed by acid deposition. It is likely that these trees have been harmed by both the acidity of the deposition as well as by the nitrate and sulfate ions.
People are not harmed by direct contact with precipitation at the acidities commonly experienced in the United States or elsewhere in the world because human skin is a sufficiently robust barrier. Human health is more affected by the precursors to acid deposition such as sulfur dioxide and nitrogen oxides.
Acid deposition can, however, harm human-