module 48 Pollution Control Measures
In our discussion of energy and energy choices, we saw that sustainability is best achieved by considering conservation and efficiency first. Similarly, if we can address the problems of pollution by seeking ways to avoid creating it in the first place, we will require less energy and fewer resources to clean it up. Preventing pollution is usually much less expensive and energy intensive then controlling it. Unfortunately, pollution prevention is not always possible.
Learning Objectives
After reading this module, you should be able to
explain strategies and techniques for controlling sulfur dioxide, nitrogen oxides, and particulate matter.
describe innovative pollution control measures.
Pollution control includes prevention, technology, and innovation
As with other types of pollution, the best way to decrease air pollution emissions is to avoid them in the first place. This can be achieved through the use of fuels that contain fewer impurities. Coal and oil, for example, both occur naturally with different sulfur concentrations and are available for purchase at a variety of sulfur concentrations. In addition, during refining and processing, the concentration of sulfur can be reduced in both fuels.
Use of a low-
Control of Sulfur and Nitrogen Oxide Emissions
Sulfur and nitrogen oxides are common air pollutants in the United States and they cause a variety of environmental problems, including acid deposition that we described in the previous module. A substantial number of air pollution control measures have been directed toward sulfur and nitrogen oxides. Sulfur dioxide emissions from coal exhaust can be reduced by a process known as fluidized bed combustion. In this process, granulated coal is burned in close proximity to calcium carbonate. The heated calcium carbonate absorbs sulfur dioxide and produces calcium sulfate, which can be used in the production of gypsum wallboard, also known as sheetrock, for houses. Some of the sulfur oxide that does escape the combustion process can be captured by other methods after combustion.
The atmosphere of Earth is 78 percent nitrogen gas and, as a result, nitrogen oxides are produced in virtually all combustion processes. Hotter burning conditions and the presence of oxygen allow proportionally more nitrogen oxide to be generated per unit of fuel burned. In order to reduce nitrogen oxide emissions, burn temperatures must be reduced and the amount of oxygen must be controlled—
Nitrogen oxide emissions from automobiles have also been reduced significantly in the United States over the last 35 years. Beginning in 1975, all new automobiles sold in the United States were required to include a catalytic converter, which reduces the nitrogen oxide and carbon monoxide emissions. In order to operate properly, the precious metals in the catalytic converter (mostly platinum and palladium) cannot be exposed to lead. Therefore gasoline could no longer contain lead. As FIGURE 46.6 illustrates, the change in gasoline formulation caused a significant reduction in emissions of lead from automobiles and in lead concentrations in the atmosphere. At the same time, improvements in the combustion technology of power plants and factories also reduced emissions of nitrogen oxides.
Control of Particulate Matter
The removal of particulate matter is the most common means of pollution control. Sometimes the process of removing particulate matter also removes sulfur. There are a variety of methods used to remove particulate matter. The simplest is gravitational settling, which relies on gravity as the exhaust travels through the smokestack. The particles simply settle out to the bottom. The ash residue that accumulates must be disposed of in a landfill. Depending on the fuel that was burned, the ash may contain sufficiently high concentrations of metals that require special disposal. This subject will be covered in more detail in Chapter 16 on solid waste.
Pollution control devices remove particulate matter and other compounds after combustion. Each has its advantages and disadvantages and all of them use energy—
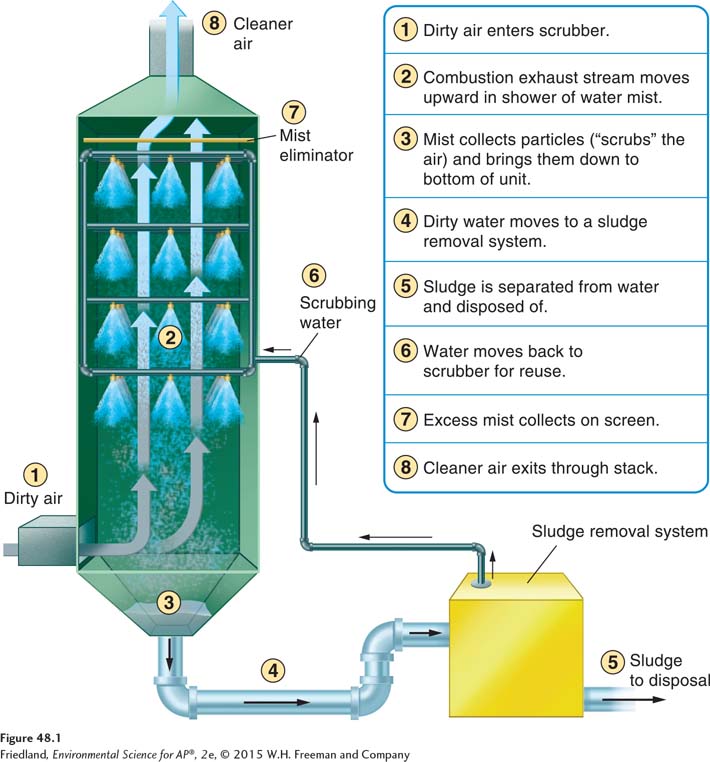
Devices such as the electrostatic precipitator and the scrubber have helped reduce pollution significantly before it is released into the atmosphere. It is much harder—
Smog Reduction
We have seen that many cities in the United States and around the world continue to have smog problems. Because the main component of photochemical smog—
Around the world, people are implementing innovative pollution control measures
A number of cities around the world, including those in China, Mexico, and England, have taken innovative and often controversial measures to reduce smog levels. Municipalities have passed measures, for example, to reduce the amount of gasoline spilled at gasoline stations, restrict the evaporation of dry-
Since cars are responsible for large emissions of nitrogen oxides and VOCs in urban areas, and these two compounds are the major contributors to smog formation, some municipalities have tried to achieve lower smog concentrations by restricting automobile use. A number of cities, including Mexico City, have instituted plans permitting automobiles to be driven only every other day—

Limiting automobile use has also helped to reduce other air pollutants. Carpool lanes, available in many areas, reduce the number of cars on the road by encouraging two or more people to share one vehicle. Improving the quality and accessibility of public transportation encourages people to leave their cars at home. A number of cities in England, including London, have been experimenting with charging individual user fees (tolls) for the use of roads at certain times of the day or within certain parts of a city as a way to reduce automobile traffic. Road user fees have been proposed for cities in the United States, including New York City, but none has yet been implemented.
In 1990 and again in 1995, scientists, policy makers, and academics collaborated on amendments to the Clean Air Act that would allow the free market to determine the least expensive ways to reduce emissions of sulfur dioxide. The free-
One of the most innovative aspects of the Clean Air Act amendments was the provision for the buying and selling of allowances that authorized the owner to release a certain quantity of sulfur. Each allowance authorizes a power plant or industrial source to emit one ton of SO2 during a given year. Sulfur allowances are awarded annually to existing sulfur emitters proportional to the amounts of sulfur they were emitting before 1990, and the emitters are not allowed to emit more sulfur than the amount for which they have permits. At the end of a given year, the emitter must possess a number of allowances at least equal to its annual emissions. In other words, a facility that emits 1,000 tons of SO2 must possess at least 1,000 allowances that are usable in that year. Facilities that emit quantities of SO2 above their allowances must pay a financial penalty.
Sulfur allowances can be bought and sold on the open market by anyone. If emitters wanted to exceed their allowance level—