module 49 Stratospheric Ozone Depletion
We have seen that tropospheric or ground-
Learning Objectives
After reading this module, you should be able to
explain the benefits of stratospheric ozone and how it forms.
describe the depletion of stratospheric ozone.
explain efforts to reduce ozone depletion.
Stratospheric ozone is beneficial to life on Earth
The Sun radiates energy at many different wavelengths, including the ultraviolet range (see FIGURE 5.1 on page 45). The ultraviolet wavelengths are further classified into three groups: UV-
As we saw in Chapter 4, a layer of ozone in the stratosphere (see FIGURE 9.1 on page 106) absorbs ultraviolet radiation, filtering out harmful UV rays from the Sun. It is easy to confuse stratospheric ozone with tropospheric, or ground-
Formation of Stratospheric Ozone
When solar radiation strikes O2 in the stratosphere, 16 to 50 km (10–
In the first step, UV-
O2 + UV-
This happens to only a few oxygen molecules at any given time. The vast majority of the oxygen in the atmosphere remains in the form O2.
In the second step, a free oxygen atom (O) produced in the first reaction encounters an oxygen molecule, and they form ozone.
O + O2 → O3
Both UV-
O3 + UV-
Thus formation of ozone in the presence of sunlight and its subsequent breakdown is a cycle that can occur indefinitely as long as there is UV energy entering the atmosphere. Under normal conditions, the amount of ozone in the stratosphere remains at steady state.
Breakdown of Stratospheric Ozone
We rely on refrigeration to keep our foods safe and edible, and on air conditioning to keep us comfortable in hot weather. For many years, the same chemicals that made refrigeration and air conditioning possible were also used in a host of other consumer items, including aerosol spray cans and products such as Styrofoam. These chemicals, called chlorofluorocarbons, or CFCs, were considered essential to modern life, and producing them was a multibillion-
CFCs introduce chlorine (Cl) into the stratosphere. When chlorine is present, it can attach to an oxygen atom in an ozone molecule, thereby breaking the bond between that atom and the molecule and forming chlorine monoxide (ClO) and O2:
O3 + Cl → ClO + O2
Subsequently, the chlorine monoxide molecule reacts with a free oxygen atom, which pulls the oxygen from the ClO to produce free chlorine again:
ClO + O → Cl + O2
When we consider these reactions together, we see that chlorine starts out and ends up as a free Cl atom. In contrast, an ozone molecule and a free oxygen atom are converted into two oxygen molecules. A substance that aids a reaction but does not get used up itself is called a catalyst. A single chlorine atom can catalyze the breakdown of as many as 100,000 ozone molecules until finally one chlorine atom finds another and the process is stopped. In the process, the ozone molecules are no longer available to absorb incoming UV-
Humans have contributed to significant destruction of the ozone layer
Ozone formation and ozone destruction have occurred for many years. But the use of CFCs as refrigerants starting in the 1920s and their increased use since that time led to the more rapid destruction of stratospheric ozone. After decades of CFC use, its effects became apparent.
Depletion of the Ozone Layer
In the mid-
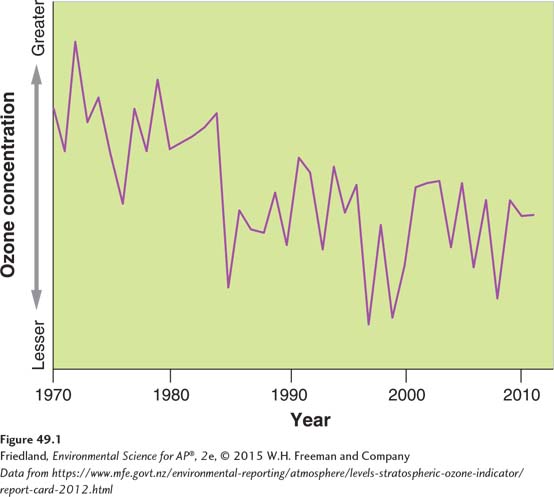
Researchers also determined that, in the Antarctic, ozone depletion was seasonal: Each year the depletion occurred from roughly August through November (late winter through early spring in the Southern Hemisphere).The depletion caused an area of severely reduced ozone concentrations over most of Antarctica, creating what has come to be called the “ozone hole.” A depletion of ozone also occurs over the Arctic in January through April, but it is not as severe, varies more from year to year, and does not cause a “hole” as in the Antarctic.
The cause of the formation of the ozone hole, which has received a great deal of media attention and has been studied intensively, is complex. It appears that extremely cold weather conditions during the polar winter cause a buildup of ice crystals mixed with nitrogen oxide. This in turn provides the perfect surface for the formation of the stable molecule Cl2, which accumulates as atmospheric chlorine interacts with the ice crystals. When the Sun reappears in the spring, UV radiation breaks down this molecule into Cl again, which in turn catalyzes the destruction of ozone as described above. Because almost no ozone forms in the dark of the polar winter, a large “hole” occurs. Only after the temperatures warm up and the chlorine gets diluted by air coming from outside the polar region does the hole diminish. In contrast, the overall global trend of decreasing stratospheric ozone concentration is not related to temperature, but is caused by the breakdown reactions described earlier that result from increased concentrations of chlorine in the atmosphere.
Decreased stratospheric ozone has increased the amount of UV-
Efforts to reduce ozone depletion have been effective
In response to the decrease in stratospheric ozone, 24 nations in 1987 signed the Montreal Protocol on Substances That Deplete the Ozone Layer. This was a commitment to reduce CFC production by 50 percent by the year 2000. It was the most far-
Because of these efforts, the concentration of chlorine in the stratosphere has stabilized at about 5 ppb (parts per billion) and should fall to about 1 ppb by 2100. The chlorine concentration reduction process is slow because CFCs are not easily removed from the stratosphere and in some recent years ozone depletion has continued to reach record levels. However, with the leveling off of chlorine concentrations, stratospheric ozone depletion should decrease in subsequent decades. The number of additional skin cancers should eventually decrease as well, although this effect will take some time due to the long time it takes for these cancers to develop.