module 57 Toxicology and Chemical Risks
The complexity of the biological risks humans face is matched by the complexity of chemical risks. Our modern society has developed an incredible array of chemicals to improve human health and food production, including pharmaceuticals, insecticides, herbicides, and fungicides. We have also seen that chemical by-
Learning Objectives
After reading this module you should be able to
identify the major types of harmful chemicals.
explain how scientists determine the concentrations of chemicals that harm organisms.
Many types of chemicals can harm organisms
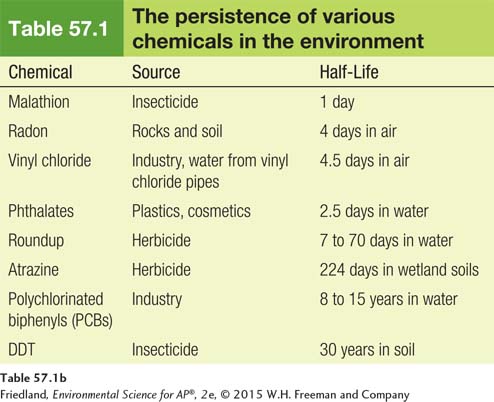
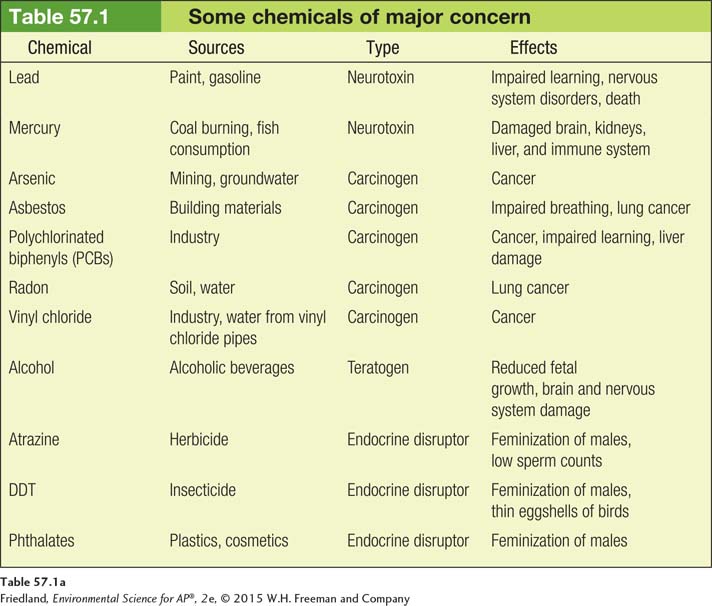
Chemicals can have many different effects on organisms, and some of the most harmful are common in our environment; TABLE 57.1 lists those of current concern. They can be grouped into five categories: neurotoxins, carcinogens, teratogens, allergens, and endocrine disruptors.
Neurotoxins
Neurotoxin A chemical that disrupts the nervous systems of animals.
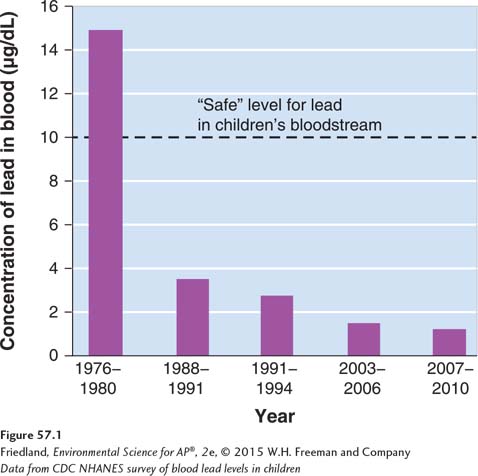
Neurotoxins are chemicals that disrupt the nervous systems of animals. Many insecticides, for example, are neurotoxins that interfere with an insect’s ability to control its nerve transmissions. Insects and other invertebrates are highly sensitive to neurotoxin insecticides. These animals can become completely paralyzed, cannot obtain oxygen, and quickly die. Other important neurotoxins include lead and mercury. As we discussed in Chapters 14 and 15, lead and mercury are very harmful heavy metals that can damage the human kidneys, brain, and nervous system. As shown in FIGURE 57.1, since the federal government required a gradual elimination of lead in gasoline and paint in the 1970s, lead exposure in the United States has declined sharply. However, lead contamination in children remains a serious problem in low-
Carcinogens
Carcinogen A chemical that causes cancer.
Mutagen A type of carcinogen that causes damage to the genetic material of a cell.
Carcinogens are chemicals that cause cancer. Carcinogens cause cell damage and lead to uncontrolled growth of these cells either by interfering with the normal metabolic processes of the cell or by damaging the genetic material of the cell. Carcinogens that cause damage to the genetic material of a cell are called mutagens (although not all mutagens are carcinogens). Some of the most well-
Teratogens
Teratogen A chemical that interferes with the normal development of embryos or fetuses.

Teratogens are chemicals that interfere with the normal development of embryos or fetuses. One of the most infamous teratogens was the drug thalidomide, prescribed to pregnant women during the late 1950s and early 1960s to combat morning sickness. Sadly, tens of thousands of these mothers around the world gave birth to children with defects before the drug was taken off the market in 1961 (FIGURE 57.2). One of the most common modern teratogens is alcohol. Excessive alcohol consumption reduces the growth of the fetus and damages the brain and nervous system of the fetus, a condition known as fetal alcohol syndrome. This is why physicians recommend that women not consume alcoholic beverages while they are pregnant.
Allergens
Allergen A chemical that causes allergic reactions.
Allergens are chemicals that cause allergic reactions. Although allergens are not pathogens, allergens are capable of causing an abnormally strong response from the immune system. In some cases, this response can cause breathing difficulties and even death. Typically, a given allergen only causes allergic reactions in a small fraction of people. Some common chemicals that cause allergic reactions include the chemicals naturally found in peanuts and milk and several drugs including penicillin and codeine.
Endocrine Disruptors
Endocrine disruptor A chemical that interferes with the normal functioning of hormones in an animal’s body.
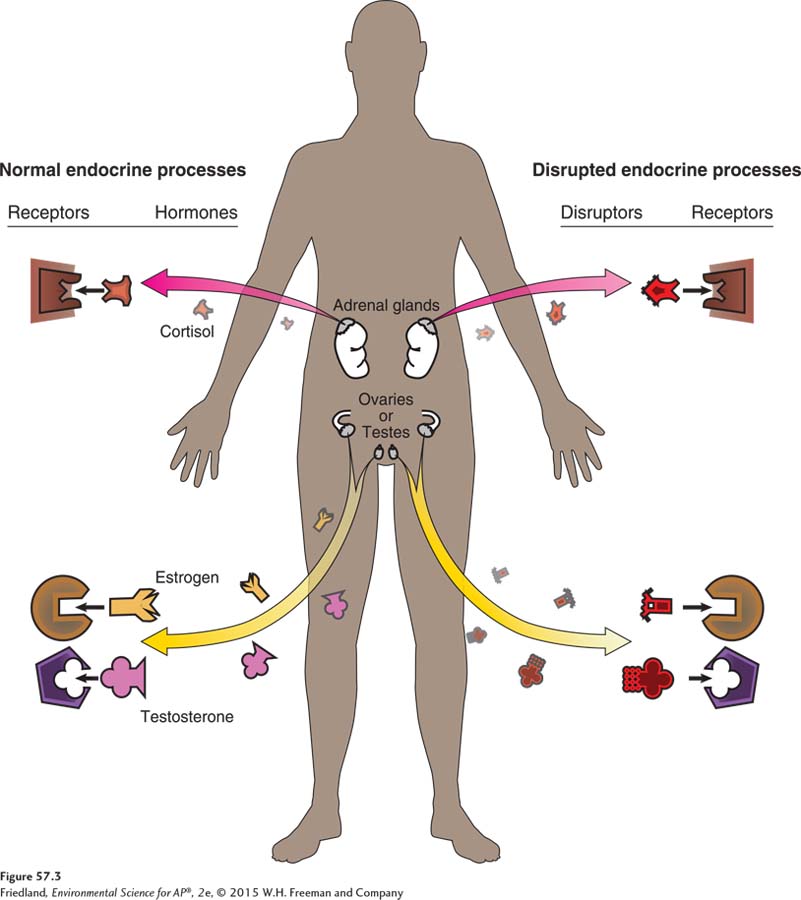
Endocrine disruptors are chemicals that interfere with the normal functioning of hormones in an animal’s body. Hormones are normally manufactured in the endocrine system and released into the bloodstream in very low concentrations. As the hormones move through the body, they bind to specific cells. Binding stimulates the cell to respond in a way that regulates the functioning of the body including growth, metabolism, and the development of reproductive organs. As FIGURE 57.3 shows, an endocrine disruptor can bind to receptive cells and cause the cell to respond in ways that are not beneficial to the organism.
One high-
Scientists can determine the concentrations of chemicals that harm organisms
To assess the risk a chemical poses, we need to know the concentrations that cause harm. Scientists have three techniques to determine harmful concentrations: dose-
Dose-
Dose-
Acute study An experiment that exposes organisms to an environmental hazard for a short duration.
Chronic study An experiment that exposes organisms to an environmental hazard for a long duration.
Dose-

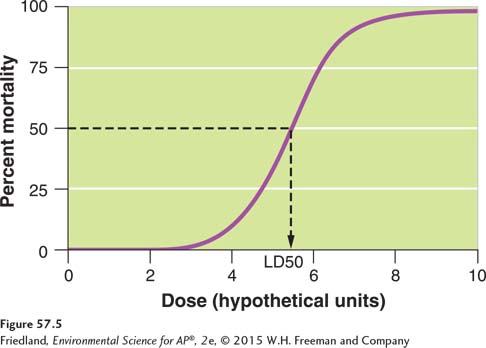
Dose-
LD50 The lethal dose of a chemical that kills 50 percent of the individuals in a dose-
To compare the harmful effects of different chemicals scientists measure the LD50, which is an abbreviation for the lethal dose that kills 50 percent of the individuals in a dose-
Although the vast majority of toxicology studies are only conducted for a few days, chronic studies will often last from the time an organism is very young to when it is old enough to reproduce. For some species such as fish, chronic experiments can take several months. The goal of chronic studies is to examine the long-
Sublethal effect The effect of an environmental hazard that is not lethal, but which may impair an organism’s behavior, physiology, or reproduction.
ED50 The effective dose of a chemical that causes 50 percent of the individuals in a dose-
Not all dose-
Testing Standards
In the United States, chemicals that affect humans and other species are regulated by the Environmental Protection Agency (EPA). The Toxic Substances Control Act of 1976 gives the EPA the authority to regulate many chemicals, but does not include food, cosmetics, and pesticides. Pesticides are regulated under a separate law—
Because no chemical can be tested on every one of the approximately 10 million species of organisms on Earth, scientists have devised a system of testing a few species—
You might have noticed that the groups of tested animals do not include amphibians or reptiles. Unfortunately, the standards for testing chemicals were set up before there was much interest in protecting amphibians and reptiles. Currently, test results from fish are used to represent aquatic amphibians and reptiles, whereas test results from birds are used to represent terrestrial amphibians and reptiles. Because amphibians and reptiles are now experiencing population declines throughout the world, there is increased interest in requiring tests on species from these two groups as well.
Using the LD50 and ED50 values from dose-
The regulatory agencies, however, are much more conservative in setting concentrations for humans. Scientists determine the LD50 or ED50 values for rats or mice and then divide by 10 to determine a safe concentration for rats and mice. This value is divided by 10 again to reflect that rats and mice may be less sensitive to a chemical than humans. Finally, this value is often divided by 10 again to ensure an extra level of caution. In short, the LD50 and ED50 values obtained from rats and mice are divided by 1,000 to set the safe values for humans. “Do the Math: Estimating LD50 Values and Safe Exposures” on page 610 shows you how to make this calculation.
Retrospective versus Prospective Studies
Estimating the effects of chemicals on humans is a major challenge. We have seen that one approach is to conduct dose-
Retrospective study A study that monitors people who have been exposed to an environmental hazard at some time in the past.
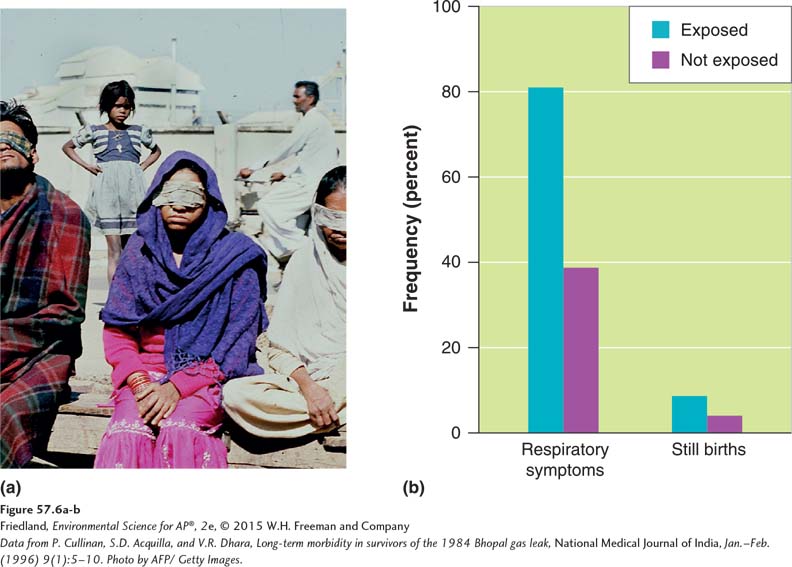
Retrospective studies monitor people who have been exposed to a chemical at some time in the past. In such studies, scientists identify a group of people who have been exposed to a potentially harmful chemical and a second group of people who have not been exposed to the chemical. Both groups are then monitored for many years to see if the exposed group experiences more health problems than the unexposed group. In 1984, for example, there was an accidental release of methyl isocyanate gas from a Union Carbide pesticide factory in Bhopal, India (FIGURE 57.6a). More than 36,000 kg (80,000 pounds) of hazardous gas spread through the city of 500,000 inhabitants. An estimated 2,000 people died that night and another 15,000 died later from effects related to the exposure. For more than 2 decades scientists have been monitoring many citizens of Bhopal to determine if survivors of the accident have developed any additional health problems. The retrospective studies have found that approximately 100,000 people are still suffering illnesses from the accidental exposure to the gas. The survivors have higher rates of genetic abnormalities, infant mortality, kidney failure, and learning disabilities. As shown in FIGURE 57.6b, they also have higher rates of respiratory problems and stillbirths.
Prospective study A study that monitors people who might become exposed to harmful chemicals in the future.
Synergistic interaction A situation in which two risks together cause more harm than expected based on the separate effects of each risk alone.
In contrast to retrospective studies, prospective studies monitor people who might become exposed to harmful chemicals in the future. In this case, scientists might select a group of 1,000 participants and ask them to keep track of the food they eat, the tobacco they use, and the alcohol they drink over a period of several decades. As time passes, the researchers can determine if the habits of the participants are associated with any future health problems. Prospective studies can be quite challenging because a participant’s habits, such as tobacco use, can also be associated with many other risk factors, such as socioeconomic status. Of particular concern is when multiple risks cause synergistic interactions, in which two risks together cause more harm than expected based on the separate effects of each risk alone. For example, the health impact of a carcinogen such as asbestos can be much higher if an individual also smokes tobacco.
Studies of lead in children are often prospective. In one study, researchers at Harvard University looked at the effects of lead on children’s intelligence by following 276 children in Rochester, New York, from 6 months to 5 years of age. IQ tests are reliable at the age of 5. In addition to lead exposure, the researchers also accounted for other factors that might affect childhood IQ including the mother’s IQ, exposure to tobacco, and the intellectual environment of their homes. After controlling for these other factors, the researchers found that among children who had been exposed to lead in the environment—
Factors That Determine the Concentrations of Chemicals That Organisms Experience
Knowing the concentrations of chemicals that can harm humans or other animals is important, but it is only useful when combined with information about the concentrations that an individual might actually experience in the environment. If a chemical is quite harmful at some moderate concentration but individuals only experience lower concentrations of that chemical, we might not be particularly concerned. Therefore, to identify and understand the effects of chemical concentrations that organisms experience, we need to know something about how the chemicals behave in the environment.
Routes of Exposure
Route of exposure The way in which an individual might come into contact with an environmental hazard.
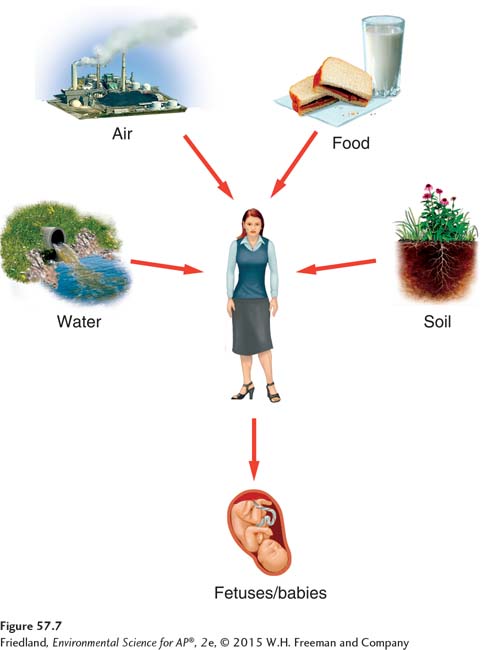
The ways in which an individual might come into contact with a chemical are known as routes of exposure. As FIGURE 57.7 illustrates, the full range of possibilities is complex because it includes potential exposures from the air, from water used for drinking, bathing, or swimming, from food, and from the environments of places where people live, work, or visit. For any particular chemical, however, the major routes of exposure are usually limited to just a few of the many possible routes. For example, bisphenol A is a chemical used in manufacturing hard plastic items such as toys, food containers, and baby bottles. Recent research has raised concerns that bisphenol A may be responsible for early puberty and increased rates of cancer. While these effects are being debated and investigated, it is clear that a child’s routes of exposure to bisphenol A are limited to toys, food containers, and baby bottles.
Solubility of Chemicals, Bioaccumulation, and Biomagnifications
Solubility How well a chemical dissolves in a liquid.
Once we know the potential routes of exposure, scientists can then determine the chemical’s solubility and its potential for bioaccumulation and biomagnification. The movement of a chemical in the environment depends in part on its solubility, which is how well a chemical can dissolve in a liquid. For example, some chemicals such as herbicides are readily soluble in water whereas others such as insecticides are much more soluble in fats and oils. When a chemical is highly soluble in water, it can be washed off surfaces, percolate into groundwater, and run off into surface waters including rivers and lakes. In contrast, chemicals that are soluble in fats and oils are not very soluble in water so they tend not to be found percolating into the groundwater or running off into surface waters. Instead, they can be found in higher concentrations bound to soils, including the benthic soils that underlie bodies of water.
Bioaccumulation An increased concentration of a chemical within an organism over time.
Chemicals that are soluble in fats and oils can also become stored in the fatty tissues of animals. For example, in Chapter 11 we mentioned that DDT accumulates in the fatty tissues of eagles and pelicans. This process, known as bioaccumulation, occurs when an organism increases the concentration of a chemical in its body over time. The process of bioaccumulation begins when an individual is exposed to small amounts of a chemical from the environment and incorporates the chemical into its tissues, typically its fat tissues. Fish, for example, are exposed to low concentrations of methyl mercury when they drink water, pass water over their gills to breathe, and consume food that contains mercury. A fish stores mercury in its fat tissues and, over time, the mercury accumulates. The rate of accumulation for any animal will depend on the concentration of the chemical in the environment, the rate at which the animal takes up each source of the chemical, the rate at which the chemical breaks down inside the animal, and the rate at which it is excreted by the animal.
Biomagnification The increase in chemical concentration in animal tissues as the chemical moves up the food chain.
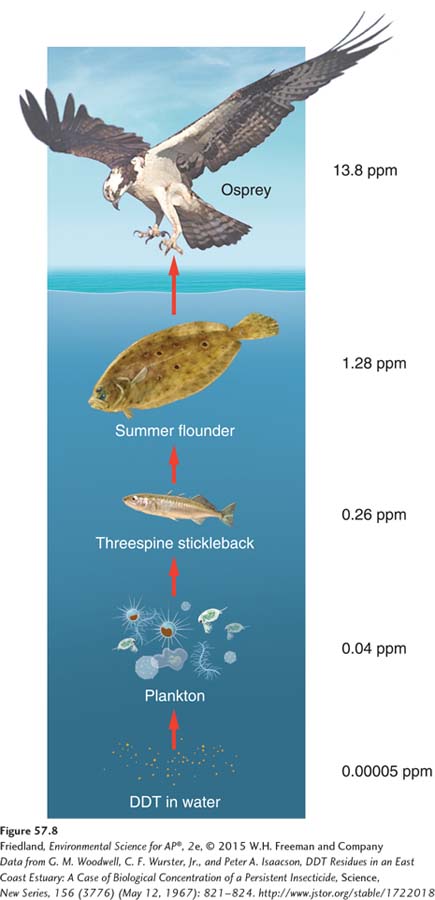
Biomagnification is the increase in chemical concentration in animal tissues as the chemical moves up the food chain. In this way, the original concentration in the environment is magnified to occur at a much higher concentration in the top predator of the community. The classic example of biomagnification is the case of DDT, an insecticide that has been widely used to kill insect pests in agriculture and to kill the mosquitoes that carry malaria and other diseases. DDT is not soluble in water, so when sprayed over water it quickly binds to particulates in the water and the underlying soil or is quickly taken up by the tiny zooplankton that act as primary consumers on algae. As we see in FIGURE 57.8, the very low concentration of DDT in the water bioaccumulates in the bodies of the zooplankton where it becomes approximately 1,000 times more concentrated. Small fish eat the zooplankton for many weeks or months and the DDT is further concentrated approximately sixfold. Large fish spend their lives eating the contaminated smaller fish and the DDT in the large fish is further concentrated approximately fivefold. Finally, fish-
Persistence
Persistence The length of time a chemical remains in the environment.
The persistence of a chemical refers to how long the chemical remains in the environment. Persistence depends on a number of factors including temperature, pH, whether the chemical is in water or soil, and whether it can be degraded by sunlight or broken down by microbes. Scientists often measure persistence by observing the time needed for a chemical to degrade to half its original concentration, known as the half-