module 62 Global Climate Change and the Greenhouse Effect
In this module, we will consider the distinctions among global change, global climate change, and global warming. We will then explore the processes that underlie changes in global climates and, more specifically, global temperatures.
Learning Objectives
After reading this module you should be able to
distinguish among global change, global climate change, and global warming.
explain the process underlying the greenhouse effect.
identify the natural and anthropogenic sources of greenhouse gases.
Global change includes global climate change and global warming
Throughout this book, we have highlighted a wide variety of ways in which the world has changed as a result of a rapidly growing human population. Human activity has placed increasing demands on natural resources such as water, trees, minerals, and fossil fuels. We have also emitted growing amounts of carbon dioxide, nitrogen compounds, and sulfur compounds into the atmosphere. Our agricultural methods depend on chemicals, including fertilizers and pesticides. Finally, a growing population faces challenges of waste disposal, sanitation, and the spread of human diseases.
Global change Change that occurs in the chemical, biological, and physical properties of the planet.
Change that occurs in the chemical, biological, and physical properties of the planet is referred to as global change. As you can see in FIGURE 62.1, some types of global change are natural and have been occurring for millions of years. Global temperatures, for example, have fluctuated over millions of years. During periods of cold temperatures, Earth has experienced ice ages. In modern times, however, the rates of change have often been much higher than those that occurred historically. Many of these changes are the result of human activities, and they can have significant, sometimes cascading, effects. For example, as we saw in Chapter 17, emissions from coal-
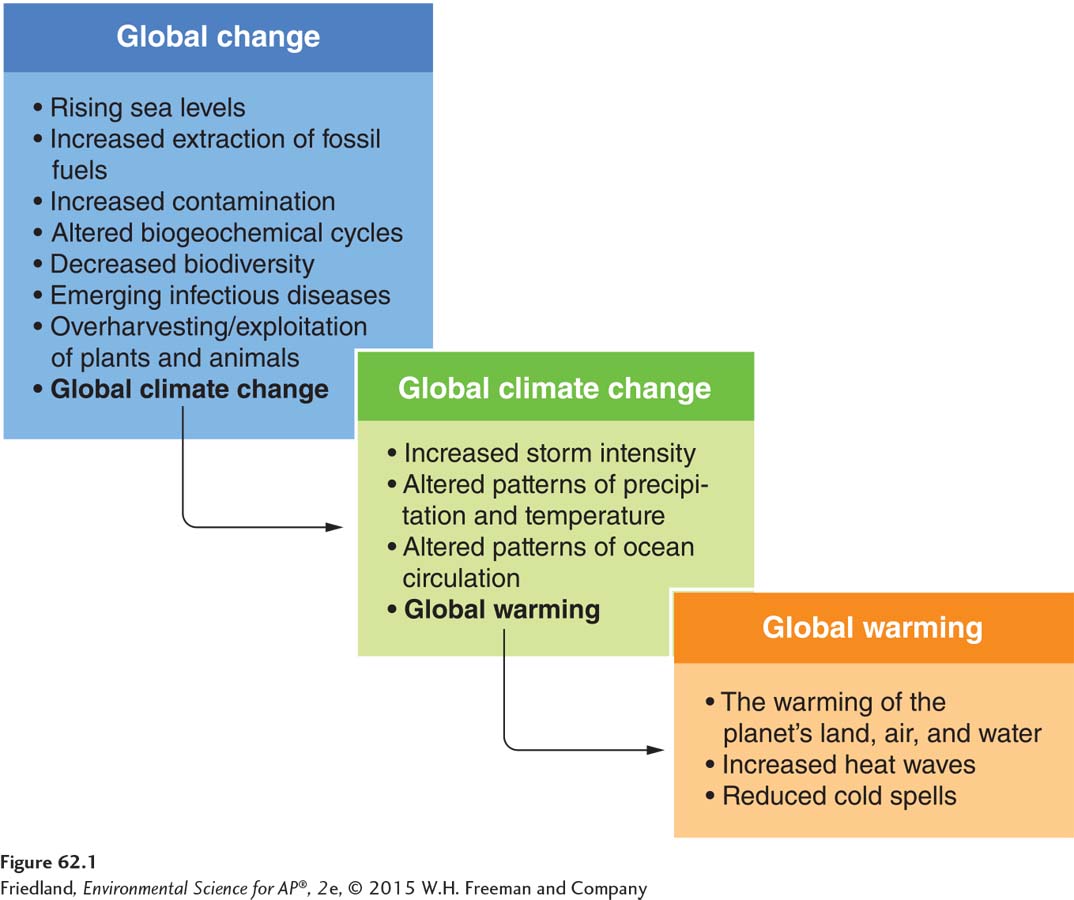
Global climate change Changes in the average weather that occurs in an area over a period of years or decades.
Global warming The warming of the oceans, land masses, and atmosphere of Earth.
One type of global change of particular concern to scientists is global climate change, which refers to changes in the average weather that occurs in an area over a period of years or decades. Changes in climate can be categorized as either natural or anthropogenic. For example, you might recall from Chapter 4 that El Niño events, which occur every 3 to 7 years, alter global patterns of temperature and precipitation (see FIGURE 11.3 on page 120). Anthropogenic activities such as fossil fuel combustion and deforestation also have major effects on global climates. Global warming refers to a specific aspect of climate change: the warming of the oceans, land masses, and atmosphere of Earth.
Solar radiation and greenhouse gases make our planet warm
The physical and biogeochemical systems that regulate temperature at the surface of Earth—
The Sun-
The ultimate source of almost all energy on Earth is the Sun. In the most basic sense, the Sun emits solar radiation that strikes Earth. As the planet warms, it emits radiation back toward the atmosphere. However, the types of energy radiated from the Sun and Earth are different (see FIGURE 5.1 on page 45). Because the Sun is very hot, most of its radiated energy is in the form of high-
Greenhouse effect Absorption of infrared radiation by atmospheric gases and reradiation of the energy back toward Earth.
Differences in the types of radiation emitted by the Sun and Earth, in combination with processes that occur in the atmosphere, cause the planet to warm. Using FIGURE 62.2, we can walk through each step of this process. As radiation from the Sun travels toward Earth, about one-
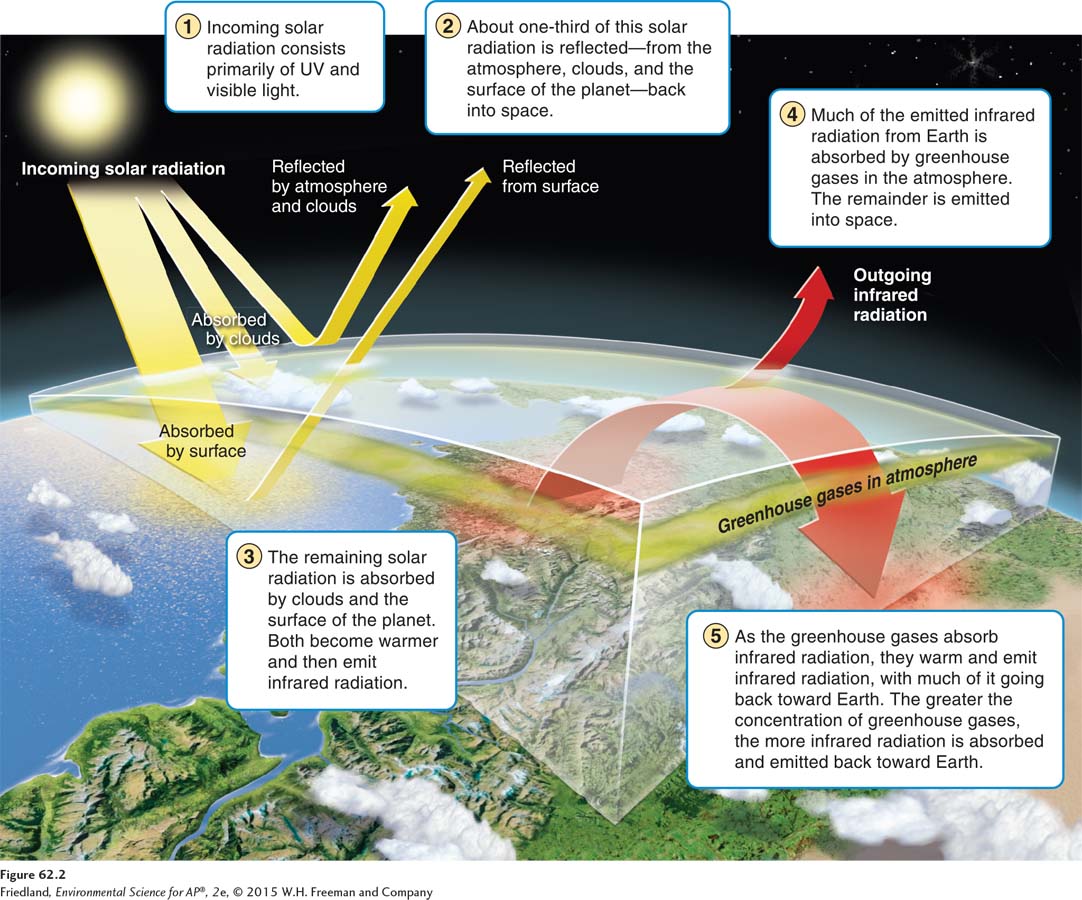
The greenhouse effect gets its name from the idea that solar radiation causes a gardener’s greenhouse to become very warm. However, the process by which actual greenhouses are warmed by the Sun involves glass windows holding in heat whereas the process by which Earth is warmed involves greenhouse gases radiating infrared energy back toward the surface of the planet.
In the Sun-
The Gases That Cause the Greenhouse Effect
Throughout the book we have seen that certain gases in the atmosphere can absorb infrared radiation emitted by the surface of the planet and radiate much of it back toward the surface. As we have seen, gases in the atmosphere that absorb infrared radiation are known as greenhouse gases.
The two most common gases in the atmosphere, N2 and O2, compose 99 percent of the atmosphere. Because these two gases do not absorb infrared radiation, they are not greenhouse gases and do not contribute to the warming of Earth. This means that greenhouse gases make up a very small fraction of the atmosphere. The most common greenhouse gas is water vapor (H2O). Water vapor absorbs more infrared radiation from Earth than any other compound, although a molecule of water vapor does not persist nearly as long as other greenhouse gases. Other important greenhouse gases include carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and ozone (O3). All of these gases have been a part of the atmosphere for millions of years, and have kept Earth warm enough to be habitable. In the case of ozone, we have seen that its effects on Earth are diverse. Ozone in the stratosphere is beneficial because it filters out harmful ultraviolet radiation. In contrast, ozone in the lower troposphere acts as a greenhouse gas and can cause increased warming of Earth. It also is an air pollutant in the lower troposphere because it can cause damage to plants and human respiratory systems. There is one other type of greenhouse gas, chlorofluorocarbons (CFCs), which does not exist naturally. It occurs in the atmosphere exclusively due to production of CFCs by humans and, as we discussed in Chapter 15, these CFCs have contributed to a hole in the ozone layer over Antarctica.
Although we commonly think of the greenhouse effect as detrimental to our environment, without any greenhouse gases the average temperature on Earth would be approximately –18°C (0°F) instead of its current average temperature of 14°C (57°F). Concern about the danger of greenhouse gases is based on our understanding that an increase in the concentration of these gases—
Greenhouse warming potential An estimate of how much a molecule of any compound can contribute to global warming over a period of 100 years relative to a molecule of CO2.
The contribution of each gas to global warming depends in part on its greenhouse warming potential. The greenhouse warming potential of a gas estimates how much a molecule of any compound can contribute to global warming over a period of 100 years relative to a molecule of CO2. In calculating this potential, scientists consider the amount of infrared energy that a given gas can absorb and how long a molecule of the gas can persist in the atmosphere. Because greenhouse gases can differ a great deal in these two factors, greenhouse warming potentials span a wide range of values. For example, water vapor has a lower potential compared with carbon dioxide. The remaining greenhouse gases have much higher values, either because they absorb more infrared radiation than a molecule of CO2 or because they persist longer in the atmosphere than CO2. TABLE 62.1 shows the global warming potential for five common greenhouse gases. Compared with CO2, the greenhouse warming potential is 25 times higher for methane (CH4), nearly 300 times higher for nitrous oxide (N2O), and up to 13,000 times higher for CFCs.
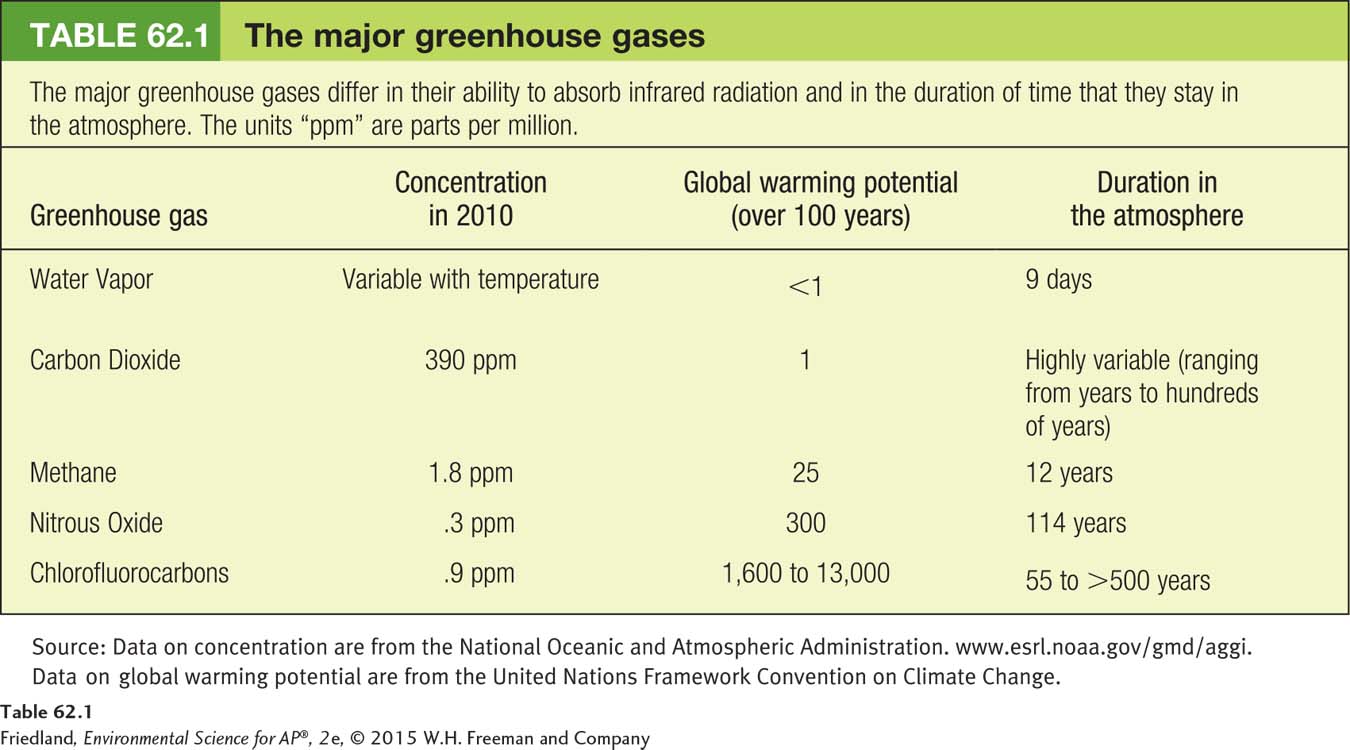
The effect of each greenhouse gas depends on both its warming potential and its concentration in the atmosphere. Although carbon dioxide has a relatively low warming potential, it is much more abundant than most other greenhouse gases, except for water vapor, which can have a concentration similar to carbon dioxide. While human activity appears to have little effect on the amount of water vapor in the atmosphere, it has caused substantial increases in the amount of the other greenhouse gases. Among these, carbon dioxide remains the greatest contributor to the greenhouse effect because its concentration is so much higher than any of the others. As a result, scientists and policy makers focus their efforts on ways to reduce carbon dioxide in the atmosphere.
Given what we now know about how greenhouse gases work, the concentrations of each gas, and how much infrared energy each gas absorbs, we can understand how changes in the concentrations of greenhouse gases can contribute to global warming. Increasing the concentration of any historically present greenhouse gas should cause more infrared radiation to be absorbed in the atmosphere, which will then radiate more energy back toward the surface of the planet and cause the planet to warm. Likewise, producing new greenhouse gases that can make their way into the atmosphere, such as CFCs, should also cause increased absorption of infrared radiation in the atmosphere and further cause the planet to warm.
Sources of greenhouse gases are both natural and anthropogenic

As we have seen, greenhouse gases include a variety of compounds such as water vapor, carbon dioxide, methane, nitrous oxide, and CFCs. These gases have natural and anthropogenic sources. After reviewing the different sources of greenhouse gases, we will discuss the relative ranks of the different anthropogenic sources.
Natural Sources of Greenhouse Gases
Natural sources of greenhouse gases include volcanic eruptions, decomposition, digestion, denitrification, evaporation, and evapotranspiration.
Volcanic Eruptions

Over the scale of geologic time, volcanic eruptions can add a significant amount of carbon dioxide to the atmosphere. Other gases and the large quantities of ash released during volcanic eruptions can also have important, short-
Decomposition and Digestion
When decomposition occurs under high-
A similar situation occurs when certain animals digest plant matter. Animals that consume significant quantities of wood or grass, including termites and grazing antelopes, require gut bacteria to digest the plant material. Because the digestion occurs in the animal’s gut, the bacteria do not have access to oxygen and methane is produced as a by-
Denitrification
As we learned in Chapter 3, nitrous oxide (N2O) is a natural component of the nitrogen cycle that is produced through the process of denitrification. Denitrification occurs in the low-
Evaporation and Evapotranspiration
As we stated earlier, water vapor is the most abundant greenhouse gas in the atmosphere and the greatest natural contributor to global warming. In Chapter 3 we examined the role of water vapor in the hydrologic cycle. (FIGURE 7.1 on page 80 shows the hydrologic cycle.) Water vapor is produced when liquid water from land and water bodies evaporates and by the evapotranspiration process of plants. Because the amount of evaporation into water vapor varies with climate, the amount of water vapor in the atmosphere can vary regionally.
Anthropogenic Sources of Greenhouse Gases
As shown in FIGURE 62.5, there are many anthropogenic sources of greenhouse gases. The most significant of these are the burning of fossil fuels, agricultural practices, deforestation, landfills, and industrial production of new greenhouse chemicals.
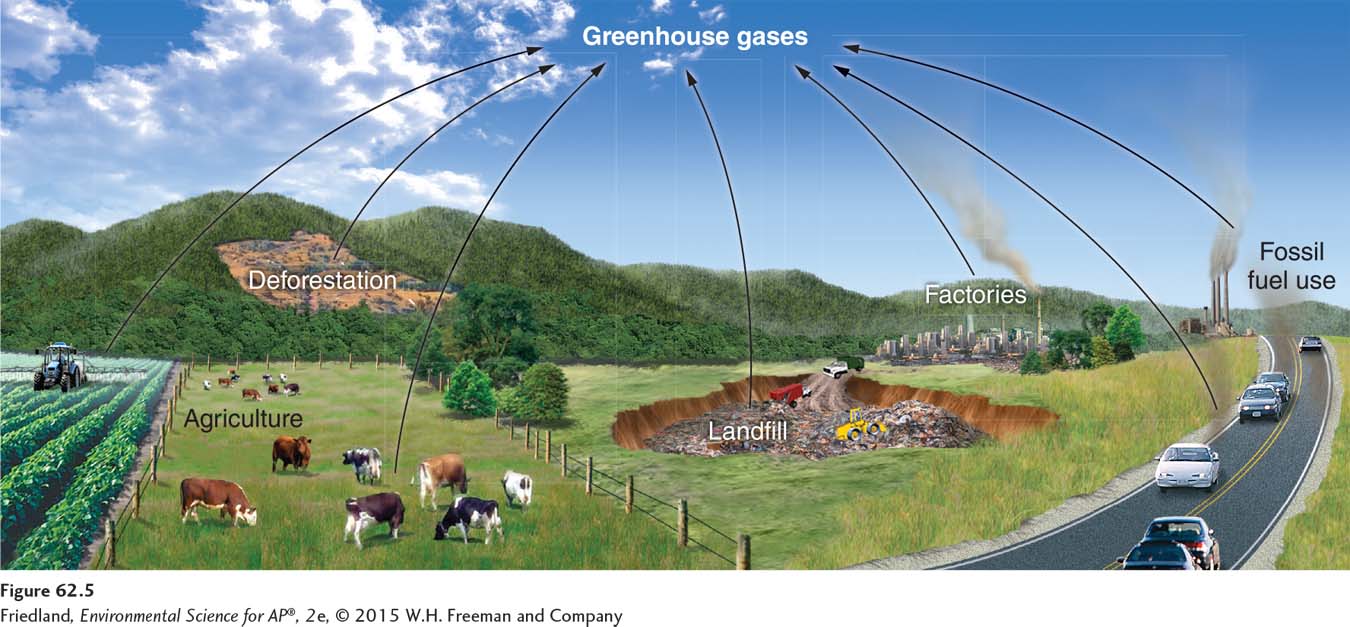
Burning Fossil Fuels
Tens to hundreds of millions of years ago, organisms were sometimes buried without first decomposing into carbon dioxide. In FIGURE 7.2 on page 83 we outlined the process by which the carbon contained in these organisms, called fossil carbon, is slowly converted to fossil fuels deep underground. When humans burn these fossil fuels, we produce CO2 that goes into the atmosphere. Because of the long time required to convert carbon into fossil fuels, the rate of putting carbon into the atmosphere by burning fossil fuels is much greater than the rate at which producers take CO2 out of the air and both the producers and consumers contribute to the pool of buried fossil carbon.
Because fossil fuels differ in how they store energy, each type of fossil fuel produces different amounts of carbon dioxide. For a given amount of energy, burning coal produces the most CO2. In comparison, burning oil produces 85 percent as much CO2 as coal, and natural gas produces 56 percent as much. As we saw in Chapter 12, from the perspective of CO2 emissions, natural gas is considered better for the environment than coal. The production of fossil fuels, such as the mining of coal, and the combustion of fossil fuels can also release methane and, in some cases, nitrous oxide.
Particulate matter may also play an important role in global warming. Although particulate matter, also known as black soot, may reflect solar radiation under some conditions, recent findings suggest that it may be responsible for up to one-
Agricultural Practices
Agricultural practices can produce a variety of greenhouse gases. Agricultural fields that are overirrigated, or those that are deliberately flooded for cultivating crops such as rice, create low oxygen environments similar to wetlands and therefore can produce methane and nitrous oxide. Synthetic fertilizers, manures, and crops that naturally fix atmospheric nitrogen—
Raising livestock can also produce large quantities of methane. Many livestock such as cattle and sheep consume large quantities of plant matter and rely on gut bacteria to digest this cellulose. As we saw in the case of termites, gut bacteria live in a low-
Deforestation
Each day, living trees remove CO2 from the atmosphere during photosynthesis, and decomposing trees add CO2 to the atmosphere. This part of the carbon cycle does not change the net atmospheric carbon because the inputs and outputs are approximately equal. However, when forests are destroyed by burning or decomposition and not replaced, as can happen during deforestation, the destruction of vegetation will contribute to a net increase in atmospheric CO2. This is because the mass of carbon that made up the trees is added to the atmosphere by combustion or decomposition. The shifting agriculture described in Chapter 11, which involves clearing forests and burning the vegetation to make room for crops, is a major source of both particulates and a number of greenhouse gases, including carbon dioxide, methane, and nitrous oxide.
Landfills
As we saw in Chapter 16, landfills receive a great deal of household waste that slowly decomposes under layers of soil. When the landfills are not aerated properly, they create a low-
Industrial Production of New Greenhouse Chemicals
The creation of new industrial chemicals often has unintended effects on the atmosphere. In Chapter 15 we looked at CFCs, the family of chemicals that serves as refrigerants used in air conditioners, freezers, and refrigerators. CFCs were used in the past until scientists discovered that they were damaging the protective ozone layer. As we discussed in Chapter 15, the nations of the world joined together to sign the Montreal Protocol on Substances That Deplete the Ozone Layer, which phased out the production and use of CFCs by 1996. Unfortunately, many of the alternative refrigerants that are less harmful to the ozone layer, including a group of gases known as hydrochlorofluorocarbons (HCFCs), still have very high greenhouse warming potentials. As a result, developed countries will phase out the use of HCFCs by 2030.
Ranking the Anthropogenic Sources of Greenhouse Gases
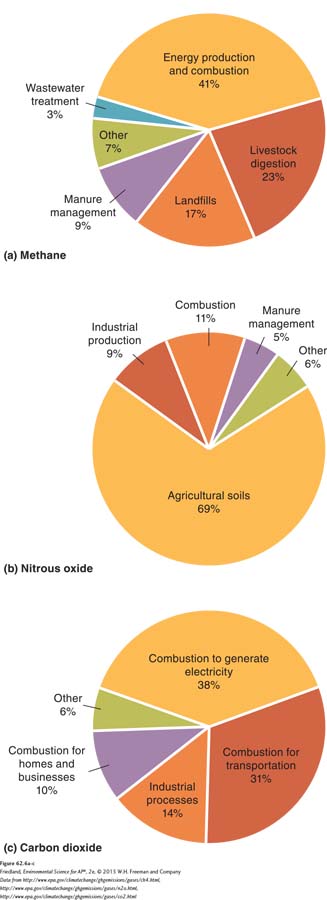
We have seen that there are multiple anthropogenic sources of greenhouse gases. What is the relative contribution of each source? FIGURE 62.6 shows the major anthropogenic sources of greenhouse gases in the United States. FIGURE 62.6a shows that the three major contributors of methane in the atmosphere are the digestive processes of livestock, landfills, and the production of natural gas and petroleum products. The major contributor of nitrous oxide, shown in FIGURE 62.6b, is agricultural soil because they receive nitrogen from synthetic fertilizers, combustion, and industrial production of fertilizers and other products. Finally, looking at the numbers for carbon dioxide in FIGURE 62.6c, we see that approximately 94 percent of all CO2 emissions come from industrial processes and the burning of fossil fuels.