200.1 1scienceapplied

What Happened to the Missing Salt?
At the beginning of the twentieth century, the City of Los Angeles needed more water for its inhabitants. As we saw at the beginning of Chapter 2, in 1913 the city designed a plan to redirect water away from Mono Lake in California. Before the Los Angeles Aqueduct was built, approximately 120 billion liters of stream water (31 billion gallons) flowed into Mono Lake in an average year. The City of Los Angeles altered the water balance of Mono Lake and at the same time caused a series of changes to the Mono Lake system that led to an increase in the salt concentration in Mono Lake.
To understand the problems at Mono Lake, ecosystem scientists had to examine water and chemical flows in natural waterways. Looking at the water and salt budgets of Mono Lake gave rise to observations, conclusions, and new studies on how human activities influence lakes. In a way, the City of Los Angeles conducted an experiment of what happens if you stop the flow of water into a terminal lake.
What is a terminal lake?
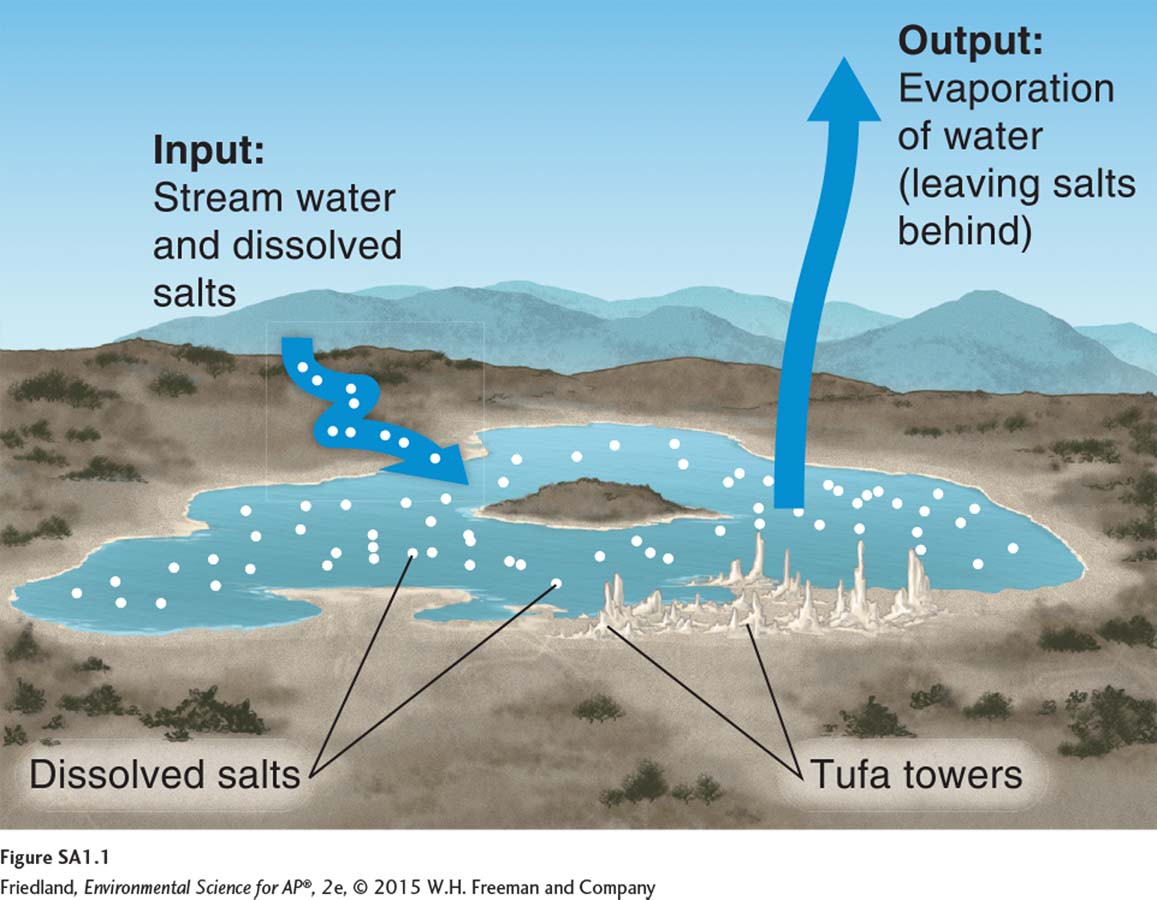
Mono Lake is a terminal lake because it is at the lowest point of the landscape: Water flows into the lake from rivers and streams and from precipitation, but does not flow out. However, in a typical year before Los Angeles began diverting water, the water level did not rise or fall at Mono Lake. The water exiting a terminal lake must balance with the water entering. If it does not, the lake will eventually either dry out or overflow its banks. But if the water level stays constant, and since Mono Lake is a terminal lake with no surface exits for liquid water, how is the water in balance? Mono Lake provides an excellent lesson in the mass balance of water: If the size of the pool does not change, then outputs must equal inputs. In this case, roughly the same amount of water that enters the lake must leave the lake. The only way this is possible is through evaporation. The input of water from streams must be equal to the output of water through evaporation.
How did the salt balance change at Mono Lake?
Although we can make the assumption that the water in Mono Lake is in steady state in a typical year, the salt balance in the lake is not. By applying some of the principles we have learned in the first two chapters, we can make observations and draw conclusions about what has probably happened at Mono Lake. The stream water that entered Mono Lake contained salt, as all natural waters do. The salt content of this water flowing into Mono Lake varied, but a typical liter of lake water averaged 50 mg of salt. Note that 50 mg/L is equivalent to 50 parts per million.
To calculate the total amount of salt that entered Mono Lake each year, we can multiply the concentration of salt, 50 mg per liter of water, by the number of liters of water flowing into the lake, before it was diverted by the City of Los Angeles: 120 billion liters per year:
50 mg/L salt × 120 billion L/year = 6 trillion mg salt/year
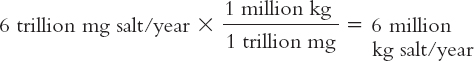
This is the annual input of salt by weight to Mono Lake. The lake today contains about 285 billion kilograms of dissolved salt, based on measurements and estimates conducted recently.
At the yearly rate of salt input we have just calculated, how long would it take to accumulate that much salt, starting with no salt in the lake? We have just determined that the salt concentration of Mono Lake increases by 6 million kilograms per year. Mono Lake contains approximately 285 billion kilograms of dissolved salts today, so at the rate of stream flow before the diversion, it would have taken about 47,500 years to accumulate that much salt:
285 billion kg ÷ 6 million kg/year = 47,500 years
Does our calculation agree with the salt in Mono Lake?
Earth scientists believe that no water has flowed out of the Mono Lake basin since it was formed about 120,000 years ago. Assuming that Earth’s climate hasn’t changed significantly over that time and that water inputs to Mono Lake have not changed drastically over that time period, what can we calculate about how much salt should be in the water of Mono Lake?
At today’s input rate, how much salt should be in the water of Mono Lake today?
6 million kg/year × 120,000 years = 720 billion kg of dissolved salt
versus 285 billion kg estimated recently.

The calculated salt contents do not match. How can we explain the discrepancy?
The lake’s towering tufa formations, prominently featured in the photograph at the beginning of Chapter 2, hold the answer: Many of the salts that entered Mono Lake over time (including calcium, sodium, and magnesium) have precipitated—
Questions
Question 200.1
1. How did Los Angeles inadvertently conduct an experiment at Mono Lake?
Question 200.2
2. What chemical principle causes terminal lakes to become more salty?
Question 200.3
3. What is the reason for the discrepancy between the two calculations of salt content in Mono Lake?
Free-
Water that flows into Mono Lake contains a much smaller concentration of salt than the water already in the lake. This inflow tends to stratify, or float on top of existing water, because fresh water is less dense that salt water. As salt from the lower layer dissolves into the upper layer, nutrients from the bottom of the lake also rise to the surface. This exchange of nutrients is critical for the growth of algae in the surface waters. Recent research suggests that the reduction of water diversion from Mono Lake had unexpected results:
In 1995, the reduction of stream diversions from Mono Lake, combined with greater than average quantities of fresh water from snowmelt runoff, led to a rapid rise in water level. The large volume of fresh water from streams led to a long-
List three potential consequences of reduced lake mixing. (3 points)
Describe two adaptive management strategies that could reduce lake stratification in Mono Lake. (3 points)
What is the chemical property of water that allows salt to dissolve? (2 points)
Why would the mixing of salt water with fresh water be considered an example of increased entropy? (2 points)