205.1 6scienceapplied

Should Corn Become Fuel?
Corn-
Ethanol proponents maintain that substituting ethanol for gasoline decreases air pollution, greenhouse gas emissions, and our dependence on foreign oil. Opponents counter that growing corn and converting it into ethanol uses more energy than we recover when we burn the ethanol for fuel. Perhaps more importantly, those energy inputs release additional carbon into the atmosphere. Are the policies that encourage corn ethanol production firmly grounded in science, or are they simply a concession to politically powerful farming interests?
Does ethanol reduce air pollution?
Ethanol (C2H6O) and gasoline (a mixture of several compounds, including heptane, C7H16) are both hydrocarbons. Under ideal conditions, in the presence of enough oxygen, burning hydrocarbons produces only water and carbon dioxide. In reality, however, gasoline-
Oxygenated fuel A fuel with oxygen as part of the molecule.
Because ethanol is an oxygenated fuel—a fuel with oxygen as part of the molecule—
Does ethanol reduce greenhouse gas emissions?
Biofuels are modern carbon, not fossil carbon. In theory, burning biofuels should not introduce additional carbon into the atmospheric reservoir because the carbon captured in growing the crops and the carbon released in burning the fuel should cancel each other out. However, agriculture in the United States depends heavily on inputs of fossil fuels to grow and process crops. Does the argument that ethanol use is carbon neutral hold up when we examine the entire ethanol production cycle from farm to filling station?
To analyze this question further, we need to examine the energy return on energy investment (EROEI) for ethanol, or how much energy we get out of ethanol for every unit of energy we put in.
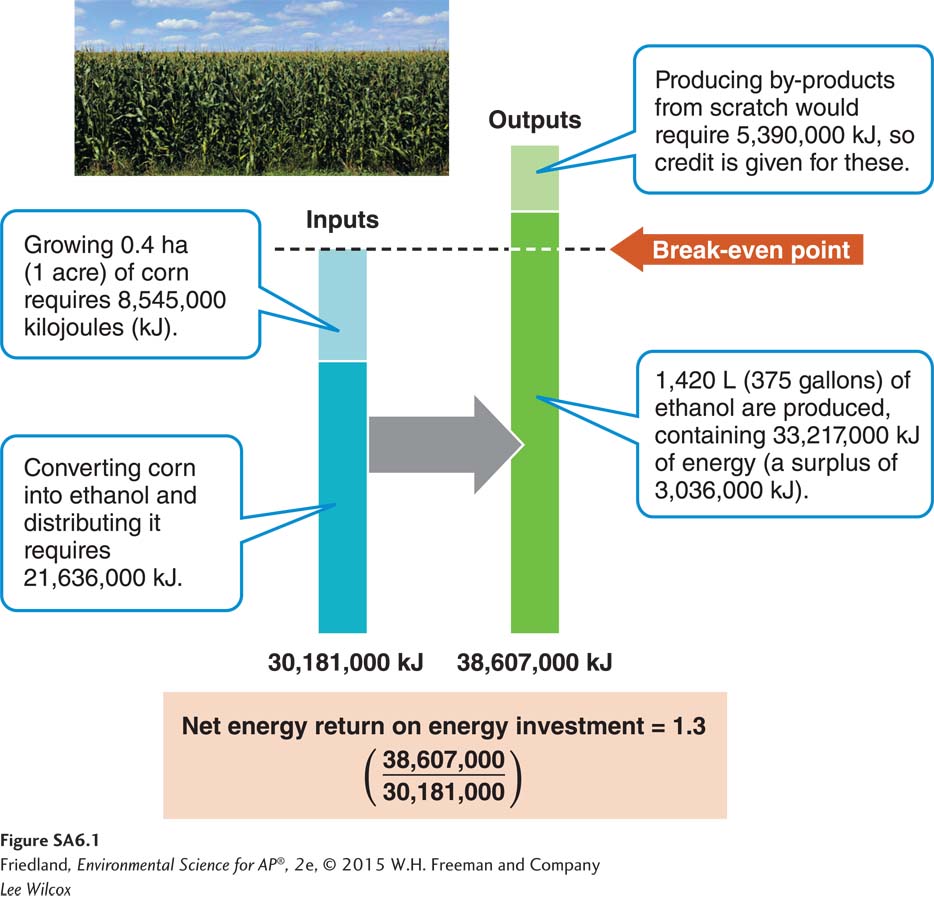
Scientists at the U.S. Department of Agriculture have analyzed this problem, examining the energy it takes to grow corn and convert it into ethanol (the inputs) and the return on this energy investment (the outputs). Energy to run farm machinery, to produce chemicals (especially nitrogen fertilizer), and to dry the corn are inputs. Additional energy is required to transport the corn, convert it into ethanol, and ship it. Ethanol is the primary output, but in the course of growing and processing the corn, several by-
Our analysis indicates that replacing part of our gasoline needs with ethanol will displace some greenhouse gases. There will be a climate benefit, but perhaps not to the extent that one would imagine because we rely so heavily on fossil fuels to support agriculture. Moreover, in the United States, the ethanol production process currently uses more coal than natural gas. Because coal emits nearly twice as much CO2 per joule of energy as natural gas (see Chapter 12), producing the ethanol may reverse many of the benefits of replacing the fossil carbon in gasoline with the modern carbon in ethanol. Quite possibly, producing ethanol with coal releases as much carbon into the atmosphere as simply burning gasoline in the first place.
Finally, we have to take into account the corn-
Does ethanol reduce our dependence on gasoline?
If there is a positive energy return on energy investment, then using ethanol should reduce the amount of gasoline we use and therefore the amount of foreign oil we must import. However, if in our attempt to use less gasoline we use more coal to produce ethanol, we might increase the release of greenhouse gases, so the overall benefit is questionable. Furthermore, if we create greater demand for a crop that until now has been primarily used as a food source, there are other implications as well.

Replacing a significant percentage of U.S. gasoline consumption with corn-
What seems more likely is that we will be able to replace some smaller fraction of gasoline consumption with biofuels. Lester Brown of the Earth Policy Institute points out, however, that the 10 bushels of corn that it takes to produce enough ethanol to fill a 95-
Indeed, in the summer of 2007, corn prices in the United States rose to $4 per bushel, roughly double the price in prior years, primarily because of the increased demand for ethanol. Since then, prices have stayed above $3 per bushel and at times have gone above $7 per bushel. People in numerous countries have had difficulty obtaining food because of these higher grain prices. In a number of years, most notably 2008 and 2011, there were food riots in Afghanistan, Algeria, Bangladesh, Egypt, Haiti, and Tunisia, among other places around the globe.
Our analysis indicates that corn-
Are there alternatives to corn ethanol?
Cellulosic ethanol An ethanol derived from cellulose, the cell wall material in plants.

Stimulating demand for corn ethanol may spur the development of another ethanol technology—
Producing cellulosic ethanol requires breaking cellulose into its component sugars before distillation. This is a difficult and expensive task because the bonds between the sugar molecules are very strong. One method of breaking down cellulose is to mix it with enzymes that sever these bonds. In 2007, the first commercial cellulosic ethanol plant was built in Iowa. At the moment, however, cellulosic ethanol is more expensive to produce than corn ethanol.
How much land would it take to produce significant amounts of cellulosic ethanol? Some scientists suggest that the impact of extensive cellulosic ethanol production would be very large, while others have calculated that, with foreseeable technological improvements, we could replace all of our current gasoline consumption without large increases in land under cultivation or significant losses in food production. Because the technology is so new, it is not yet clear who is correct. There will still be other considerations, such as the impact on biodiversity whenever land is dedicated to growing biofuels.
The good news is that many of the raw materials for cellulosic ethanol are perennial crops such as grasses. These crops do not require the high energy, fertilizer, and water inputs commonly used to grow annual plants such as corn. Furthermore, the land used to grow grass would not need to be plowed every year. Fertilizers and pesticides would also be unnecessary, eliminating the large inputs of energy needed to produce and apply them.
Algae may be an even more attractive raw material for cellulosic ethanol because its production would not need to utilize land that could otherwise be used for growing food crops or serving as a repository for carbon.
Many forms of ethanol are now being produced or are very close to being produced commercially. Production methods that use biomass grown with fewer fossil fuel inputs at a lower level of intensity on agricultural land, or without the use of land at all, are the most desirable from virtually all environmental perspectives.
What’s the bottom line?
Adding ethanol to gasoline reduces carbon monoxide formation and may reduce photochemical smog if evaporation can be controlled. But when we look at the entire “life cycle” of ethanol, from farm to tank, it’s not clear if ethanol will actually reduce greenhouse gas emissions. Because its EROEI is fairly low, and because the amount of available agricultural land is limited, corn ethanol will not replace more than a small fraction of gasoline consumption. Cellulosic ethanol shows the potential to have a significant effect on fossil fuel use, at least in part because of lower energy inputs required to obtain the raw material and convert it into a fuel. Corn should not be used for fuel to any great extent, but it may serve as a stepping-
Questions
Question 205.1
1. Legislation requiring that ethanol be added to gasoline was passed to improve air quality and reduce dependence on foreign oil. Do you think the latter goal is achieved by adding ethanol to gasoline?
Question 205.2
2. What are some of the other environmental impacts of using corn-
Free-
Write your answer to each part clearly. Support your answers with relevant information and examples. Where calculations are required, show your work.
The production of ethanol from corn is much like the production of any alcoholic beverage. Corn is harvested, ground, and cooked to form a “mash.” Yeast are added to the mash, which use the sugar to grow and reproduce. As they grow, yeast respire CO2 and produce ethanol as a waste product (i.e., through fermentation). At this point, the mixture now resembles a “corn wine.” Since ethanol has a lower boiling point than water, the fermented product can be boiled to evaporate the ethanol, which is a process known as distillation. Evaporated ethanol is then collected through condensation.
Identify six energy inputs in the process of producing corn ethanol. (2 points)
Using the answers provided in question (a), write an equation that would calculate the energy return on energy investment (EROEI) for ethanol production. (2 points)
The leftover mash consists of water and corn. Ethanol producers will often dry this mash, collect the corn, and sell it as livestock feed. Why might this livestock feed be less beneficial for livestock than unfermented corn? (2 points)
If the price of food increases around the world as a result of corn ethanol production, poorer communities may revert to subsistence energy production. Explain how this reversion could impact the total environmental benefit of ethanol production. (2 points)
Cellulosic ethanol production is similar to corn ethanol production, except producers make use of enzymes that increase the sugar content of the mash, which in turn increases the energy available for yeast to grow. Describe two benefits and two disadvantages of cellulosic ethanol production relative to corn ethanol production.
References
R.F. Service. 2013. Battle for the Barrel. Science 339:1374–
Searchinger, T. D., et al. 2009. Fixing a critical climate accounting error. Science 326:527–