module 7 The Movement of Matter
As we saw in Chapter 2, Earth is an open system with respect to energy, but a closed system with respect to matter. In the previous module, we examined how energy flows through the biosphere; it enters as energy from the Sun, moves among the living and nonliving components of ecosystems, and is ultimately emitted back into space. In contrast, matter—
The specific chemical forms that elements take determine how they cycle within the biosphere. We will look at the elements that are the most important to the productivity of photosynthetic organisms. We will begin with the hydrologic cycle—
Learning Objectives
After reading this module you should be able to
describe how water cycles within ecosystems.
explain how carbon cycles within ecosystems.
describe how nitrogen cycles within ecosystems.
explain how phosphorus cycles within ecosystems.
discuss the movement of calcium, magnesium, potassium, and sulfur within ecosystems.
The hydrologic cycle moves water through the biosphere
Biogeochemical cycle The movements of matter within and between ecosystems.
Because the movement of matter within and between ecosystems involves cycles of biological, geological, and chemical processes, these cycles are known as biogeochemical cycles. To keep track of the movement of matter in biogeochemical cycles, we refer to the components that contain the matter—
Hydrologic cycle The movement of water through the biosphere.
Water is essential to life. It makes up over one-
The Hydrologic Cycle
Transpiration The release of water from leaves during photosynthesis.
FIGURE 7.1 shows how the hydrologic cycle works. Heat from the Sun causes water to evaporate from oceans, lakes, and soils. Solar energy also provides the energy for photosynthesis, during which plants release water from their leaves into the atmosphere—
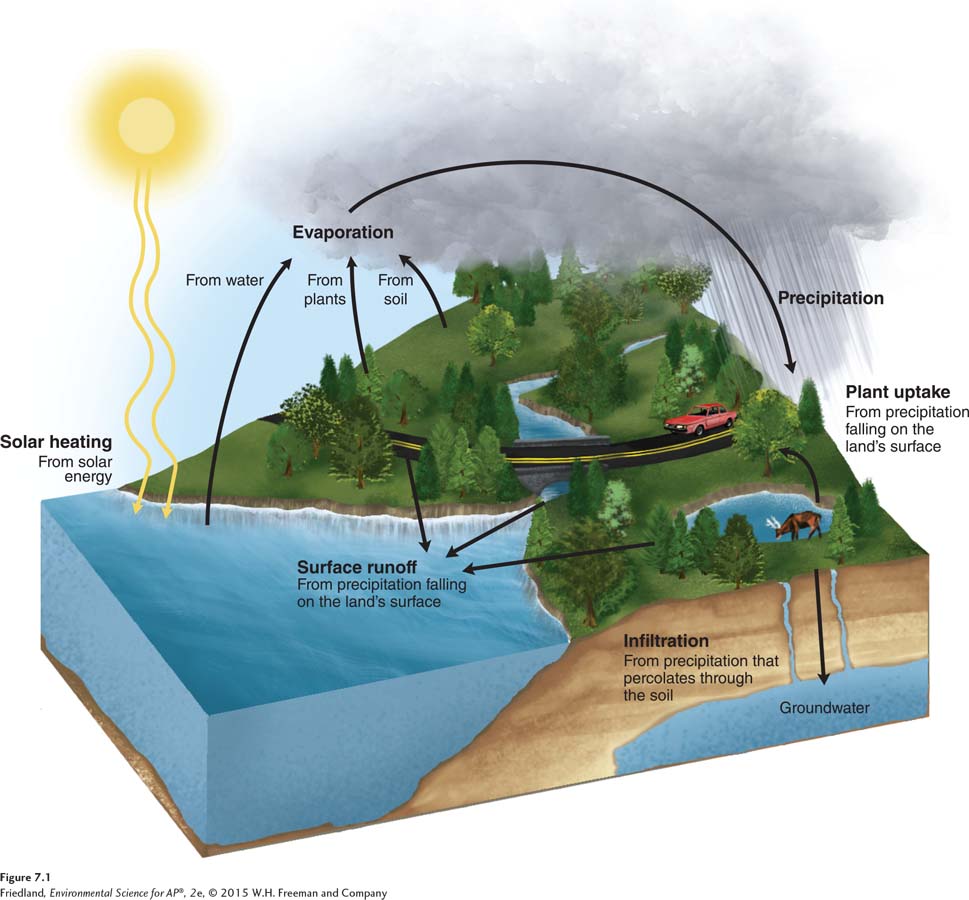
Evapotranspiration The combined amount of evaporation and transpiration.
Runoff Water that moves across the land surface and into streams and rivers.
When water falls on land, it may take one of three distinct routes. First, it may return to the atmosphere by evaporation or, after being taken up by plant roots, by transpiration. The combined amount of evaporation and transpiration, called evapotranspiration, is often used by scientists as a measure of the water moving through an ecosystem. Alternatively, water can be absorbed by the soil and percolate down into the groundwater. Finally, water can move as runoff across the land surface and into streams and rivers, eventually reaching the ocean, which is the ultimate pool of water on Earth. As water in the ocean evaporates, the cycle begins again.
The hydrologic cycle is instrumental in the cycling of elements. Many elements are carried to the ocean or taken up by organisms in dissolved form. As you read about biogeochemical cycles, notice the role that water plays in these processes.
Human Activities and the Hydrologic Cycle
Because Earth is a closed system with respect to matter, water never leaves it. Nevertheless, human activities can alter the hydrologic cycle in a number of ways. For example, harvesting trees from a forest can reduce evapotranspiration by reducing plant biomass. If evapotranspiration decreases, then runoff or percolation will increase. On a moderate or steep slope, most water will leave the land surface as runoff. That is why, as we saw at the opening of this chapter, clear-
The carbon cycle moves carbon between air, water, and land
Carbon cycle The movement of carbon around the biosphere.
Carbon is the most important element in living organisms; it makes up about 20 percent of their total body weight. Carbon is the basis of the long chains of organic molecules that form the membranes and walls of cells, constitute the backbones of proteins, and store energy for later use. Other than water, few molecules in the bodies of organisms do not contain carbon. To understand the carbon cycle, which is the movement of carbon around the biosphere, we need to examine the flows that move carbon and the major pools that contain carbon.
The Carbon Cycle
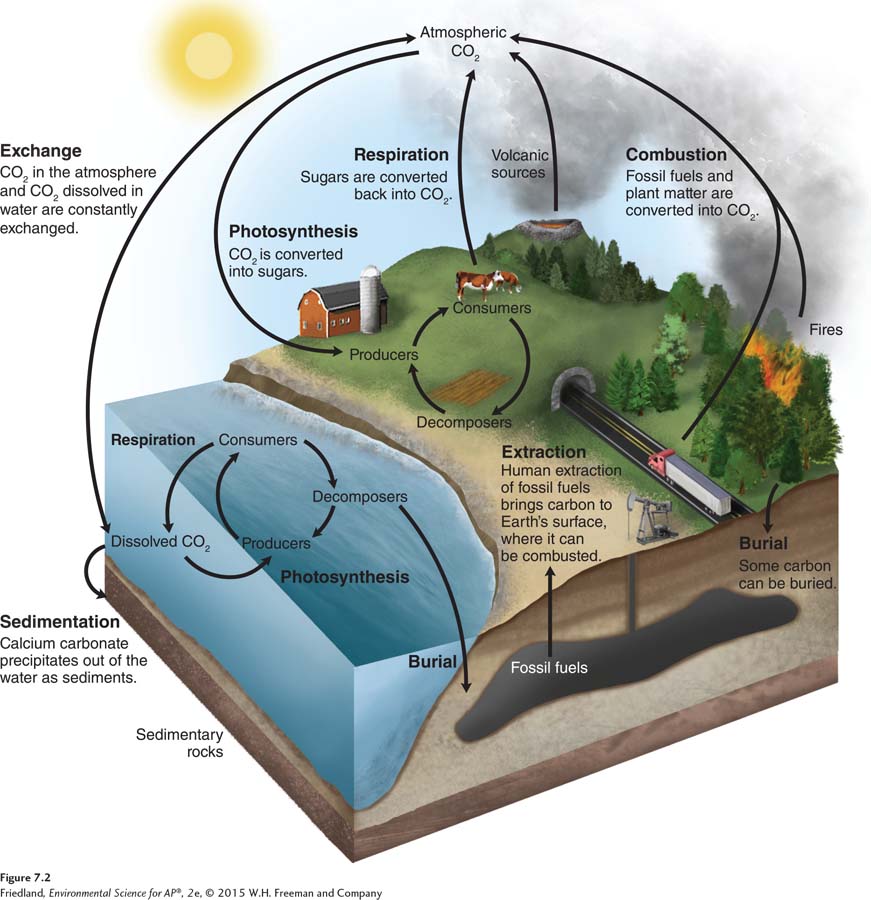
FIGURE 7.2 illustrates the seven processes that drive the carbon cycle: photosynthesis, respiration, exchange, sedimentation, burial, extraction, and combustion. These processes can be categorized as either fast or slow. The fast part of the cycle involves processes that are associated with living organisms. The slow part of the cycle involves carbon that is held in rocks, in soils, or as petroleum hydrocarbons (the materials we use as fossil fuels). Carbon may be stored in these forms for millions of years.
Photosynthesis and Respiration
Let’s take a closer look at FIGURE 7.2, which depicts the carbon cycle. When producers photosynthesize, whether on land or in the water, they take in CO2 and incorporate the carbon into their tissues. Some of this carbon is returned as CO2 when organisms respire. It is also returned after organisms die. When organisms die, carbon that was part of the live biomass pool becomes part of the dead biomass pool. Decomposers break down the dead material, which returns CO2 to the water or air via respiration and continues the cycle.
Exchange, Sedimentation, and Burial
As you can see on the left side of FIGURE 7.2, carbon is exchanged between the atmosphere and the ocean. The amount of carbon released from the ocean into the atmosphere roughly equals the amount of atmospheric CO2 that diffuses into ocean water. Some of the CO2 dissolved in the ocean enters the food web via photosynthesis by algae.
Another portion of the CO2 dissolved in the ocean combines with calcium ions in the water to form calcium carbonate (CaCO3), a compound that can precipitate out of the water and form limestone and dolomite rock via sedimentation and burial. Although sedimentation is a very slow process, the small amounts of calcium carbonate sediment formed each year have accumulated over millions of years to produce the largest carbon pool in the slow part of the carbon cycle.
A small fraction of the organic carbon in the dead biomass pool is buried and incorporated into ocean sediments before it can decompose into its constituent elements. This organic matter becomes fossilized and, over millions of years, some of it may be transformed into fossil fuels. The amount of carbon removed from the food web by this slow process is roughly equivalent to the amount of carbon returned to the atmosphere by weathering of rocks containing carbon (such as limestone) and by volcanic eruptions, so the slow part of the carbon cycle is in steady state.
Extraction and Combustion
The final processes in the carbon cycle are extraction and combustion, as are shown in the middle of FIGURE 7.2. The extraction of fossil fuels by humans is a relatively recent phenomenon that began when human society started to rely on coal, oil, and natural gas as energy sources. Extraction by itself does not alter the carbon cycle; it is the subsequent step of combustion that makes the difference. Combustion of fossil fuels by humans and the natural combustion of carbon by fires or volcanoes release carbon into the atmosphere as CO2 or into the soil as ash.
As you can see, combustion, respiration, and decomposition operate in very similar ways: All three processes cause organic molecules to be broken down to produce CO2, water, and energy. However, respiration and decomposition are biotic processes, whereas combustion is an abiotic process.
Human Impacts on the Carbon Cycle
In the absence of human disturbance, the exchange of carbon between Earth’s surface and atmosphere is in steady state. Carbon taken up by photosynthesis eventually ends up in the soil. Decomposers in the soil gradually release that carbon at roughly the same rate it is added. Similarly, the gradual movement of carbon into the buried or fossil fuel pools is offset by the slow processes that release it. Before the Industrial Revolution, the atmospheric concentration of carbon dioxide had changed very little for 10,000 years (see FIGURE 2.5 on page 12). So, until recently, carbon entering any of these pools was balanced by carbon leaving these pools.
Since the Industrial Revolution, however, human activities have had a major influence on carbon cycling. The best-
Tree harvesting is another human activity that can affect the carbon cycle. Trees store a large amount of carbon in their wood, both above and below ground. The destruction of forests by cutting and burning increases the amount of CO2 in the atmosphere. Unless enough new trees are planted to recapture the carbon, the destruction of forests will upset the balance of CO2. To date, large areas of forest, including tropical forests as well as North American and European temperate forests, have been converted into pastures, grasslands, and croplands. In addition to destroying a great deal of biodiversity, this destruction of forests has added large amounts of carbon to the atmosphere. The increases in atmospheric carbon due to human activities have been partly offset by an increase in carbon absorption by the ocean. Still, the loss of forest remains a concern.
The nitrogen cycle includes many chemical transformations
Macronutrient One of six key elements that organisms need in relatively large amounts: nitrogen, phosphorus, potassium, calcium, magnesium, and sulfur.
There are six key elements, known as macronutrients, that are needed by organisms in relatively large amounts: nitrogen, phosphorus, potassium, calcium, magnesium, and sulfur.
Limiting nutrient A nutrient required for the growth of an organism but available in a lower quantity than other nutrients.
Nitrogen is an element that organisms need in relatively high amounts. Nitrogen is used to form amino acids, the building blocks of proteins, and nucleic acids, the building blocks of DNA and RNA. Because so much of it is required, nitrogen is often a limiting nutrient for producers. A limiting nutrient is a nutrient required for the growth of an organism but available in a lower quantity than other nutrients. Therefore, a lack of nitrogen constrains the growth of the organism. Adding other nutrients, such as water or phosphorus, will not improve plant growth in nitrogen-
The Nitrogen Cycle
Nitrogen cycle The movement of nitrogen around the biosphere.
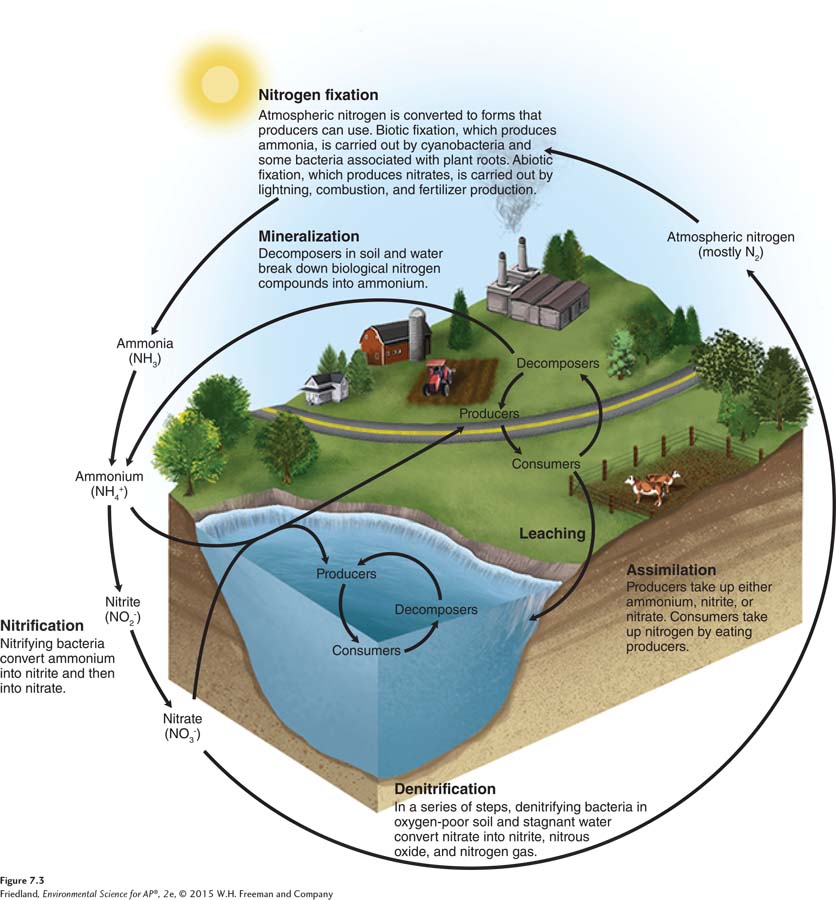
The nitrogen cycle is the movement of nitrogen around the biosphere. As nitrogen moves through an ecosystem, it experiences many chemical transformations. As shown in FIGURE 7.3, there are five major transformations in the nitrogen cycle: nitrogen fixation, nitrification, assimilation, mineralization, and denitrification.
Nitrogen Fixation
Nitrogen fixation A process by which some organisms can convert nitrogen gas molecules directly into ammonia.
Although Earth’s atmosphere is 78 percent nitrogen by volume, the vast majority of that nitrogen is in a gas form that most producers cannot use. Nitrogen fixation is the process that converts nitrogen gas in the atmosphere (N2) into forms of nitrogen that producers can use. As you can see in FIGURE 7.3, nitrogen fixation can occur through biotic or abiotic processes. In the biotic process, a few species of bacteria can convert N2 gas directly into ammonia (NH3), which is rapidly converted to ammonium (NH4+), a form that is readily used by producers. Nitrogen-
Nitrogen fixation can also occur through two abiotic pathways. N2 can be fixed in the atmosphere by lightning or during combustion processes such as fires and the burning of fossil fuels. These processes convert N2 into nitrate (NO3–), which is usable by plants. The nitrate is carried to Earth’s surface in precipitation. Humans have developed techniques for nitrogen fixation into ammonia or nitrate to be used in plant fertilizers. Although these processes require a great deal of energy, humans now fix more nitrogen than is fixed in nature. The development of synthetic nitrogen fertilizers has led to large increases in crop yields, particularly for crops such as corn that require large amounts of nitrogen.
Nitrification
Nitrification The conversion of ammonia (NH4+) into nitrite (NO2–) and then into nitrate (NO3–).
Another step in the nitrogen cycle is nitrification, which is the conversion of ammonium (NH4+) into nitrite (NO2–) and then into nitrate (NO3–). These conversions are conducted by specialized species of bacteria. Although nitrite is not used by most producers, nitrate is readily used.
Assimilation
Assimilation The process by which producers incorporate elements into their tissues.
Once producers take up nitrogen in the form of ammonia, ammonium, nitrite, or nitrate, they incorporate the element into their tissues in a process called assimilation. When primary consumers feed on the producers, some of the producer’s nitrogen is assimilated into the tissues of the consumers while the rest is eliminated as waste products.
Mineralization
Mineralization The process by which fungal and bacterial decomposers break down the organic matter found in dead bodies and waste products and convert it into inorganic compounds.
Ammonification The process by which fungal and bacterial decomposers break down the organic nitrogen found in dead bodies and waste products and convert it into inorganic ammonium (NH4).
Eventually, organisms die and their tissues decompose. In a process called mineralization, fungal and bacterial decomposers break down the organic matter found in dead bodies and waste products and convert organic compounds back into inorganic compounds. In the nitrogen cycle, the process of mineralization is sometime called ammonification because organic nitrogen compounds are converted into the inorganic ammonium (NH4+). The ammonium produced by this process can either be taken up by producers in the ecosystem or be converted into nitrite (NO2–) and nitrate (NO3–) through the process of nitrification.
Denitrification
Denitrification The conversion of nitrate (NO3–) in a series of steps into the gases nitrous oxide (N2O) and, eventually, nitrogen gas (N2), which is emitted into the atmosphere.
The final step that completes the nitrogen cycle is denitrification, which is the conversion of nitrate (NO3–) in a series of steps into the gases nitrous oxide (N2O) and, eventually, nitrogen gas (N2), which is emitted into the atmosphere. Denitrification is conducted by specialized bacteria that live under anaerobic conditions, such as waterlogged soils or the bottom sediments of oceans, lakes, and swamps.
Human Impacts on the Nitrogen Cycle
Leaching The transportation of dissolved molecules through the soil via groundwater.
Nitrogen is a limiting nutrient in most terrestrial ecosystems, so excess inputs of nitrogen can have consequences in these ecosystems. For example, nitrate is readily transported through the soil with water through leaching, a process in which dissolved molecules are transported through the soil via groundwater. In addition, adding nitrogen to soils in fertilizers ultimately increases atmospheric concentrations of nitrogen in regions where the fertilizer is applied. This nitrogen can be transported through the atmosphere and deposited by rainfall in natural ecosystems that have adapted over time to a particular level of nitrogen availability. The added nitrogen can alter the distribution or abundance of species in those ecosystems.
In one study of nine different terrestrial ecosystems across the United States, scientists added nitrogen fertilizer to some plots and left other plots unfertilized as controls. They found that adding nitrogen reduced the number of species in a plot by up to 48 percent because some species that could survive under low-
The observation that nutrients can have unintended effects on ecosystems highlights an important principle of environmental science: In ecosystems containing species that have adapted to their environments over thousands of years or longer, changes in conditions are likely to cause changes in biodiversity as well as in the movement of energy through, and the cycling of matter within, those ecosystems.
The phosphorus cycle moves between land and water
Organisms need phosphorus for many biological processes. Phosphorus is a major component of DNA and RNA as well as ATP (adenosine triphosphate), the molecule cells use for energy transfer. Required by both producers and consumers, phosphorus is a limiting nutrient second only to nitrogen in its importance for successful agricultural yields. Thus phosphorus, like nitrogen, is commonly added to soils in the form of fertilizer.
The Phosphorus Cycle
Phosphorus cycle The movement of phosphorus around the biosphere.
The phosphorus cycle is the movement of phosphorus around the biosphere. As shown in FIGURE 7.4, it primarily operates between land and water. There is no gas phase, although phosphorus does enter the atmosphere in very small amounts when dust is dissolved in rainwater or sea spray. Unlike nitrogen, phosphorus rarely changes form; it is typically found in the form of phosphate (PO43–
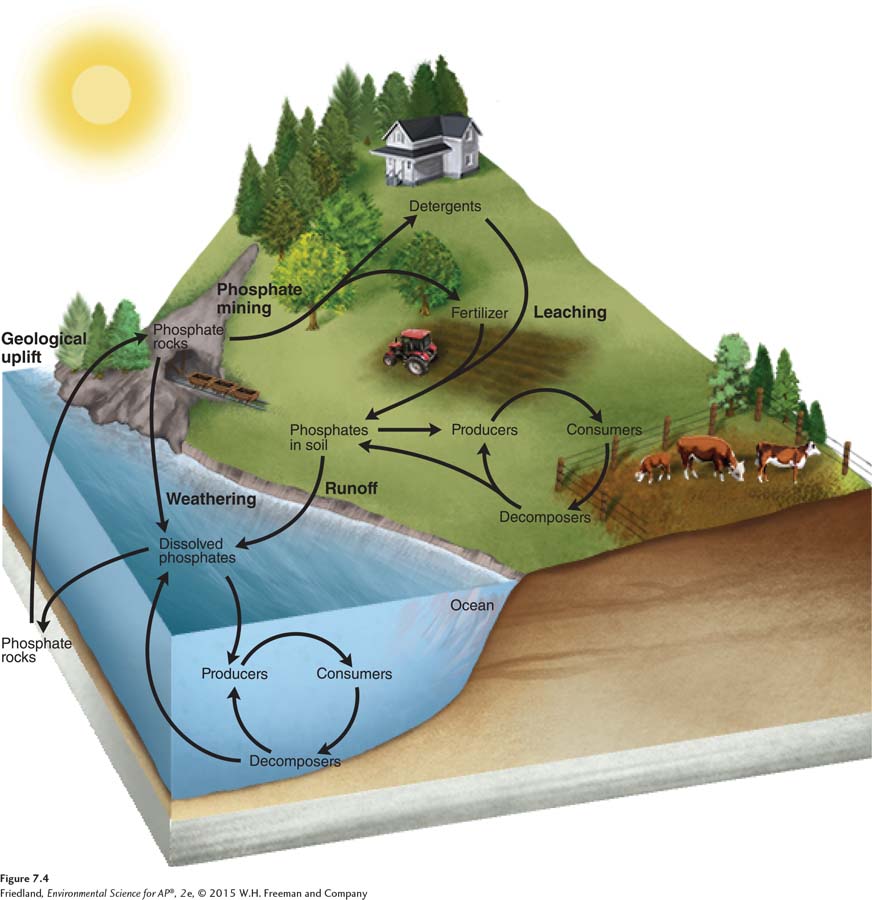
Assimilation and Mineralization
The biotic processes that affect the phosphorus cycle are not complex. Producers on land and in the water take up inorganic phosphate and assimilate the phosphorus into their tissues as organic phosphorus. The waste products and eventual dead bodies of these organisms are decomposed by fungi and bacteria, which causes the mineralization of organic phosphorus back to inorganic phosphate.
Sedimentation, Geologic Uplift, and Weathering
The abiotic processes of the phosphorus cycle involve movements between the water and the land. In water, phosphorus is not very soluble, so much of it precipitates out of solution in the form of phosphate-
Human Impacts on the Phosphorus Cycle
Algal bloom A rapid increase in the algal population of a waterway.
Hypoxic Low in oxygen.

Humans have had a dramatic effect on the phosphorus cycle. For example, humans mine the phosphate sediments from mountains to produce fertilizer. When these fertilizers are applied to lawns, gardens, and agricultural fields, excess phosphorus can leach into water bodies. Because aquatic systems are commonly limited by a low availability of phosphorus, even small inputs of leached phosphorus into these systems can greatly increase the growth of producers. Phosphorus inputs can cause a rapid increase in the algal population of a waterway, known as an algal bloom, that can quickly increase the biomass of algae in the ecosystem (FIGURE 7.5). As the algae die, decomposition consumes large amounts of oxygen. As a result, the water becomes low in oxygen, or hypoxic. Hypoxic conditions kill fish and other aquatic animals. Hypoxic dead zones occur around the world, including where the Mississippi River empties into the Gulf of Mexico.
A second major source of phosphorus in waterways is from the use of household detergents. From the 1940s through the 1990s, laundry detergents in the United States contained phosphates to make clothes cleaner. The water discharged from washing machines inadvertently fertilized streams, rivers, and lakes. Because ecological dead zones caused by excess phosphorus represent substantial environmental and economic damage, manufacturers stopped adding phosphates to laundry detergents in 1994 and 16 states banned phosphates in dishwashing detergents in 2010. In 2013, 2 more states imposed a similar ban. In Europe, the European Union recently passed a ban on phosphates in laundry detergents starting in 2013 and a ban on phosphates in dishwashing detergents starting in 2017.
In addition to causing algal blooms, increases in phosphorus concentrations can alter plant communities. We have already seen one example in Chapter 2, where we discussed the deterioration of the environment in the Florida Everglades. Because of agricultural expansion in southern Florida, the water that flows through the Everglades has experienced elevated phosphorus concentrations. This change in nutrient cycling has altered the ecosystem of the Everglades. For example, over time, cattails have become more common and sawgrass has declined. Animals that depend on sawgrass are now experiencing reduced food and habitat from this plant.
Calcium, magnesium, potassium, and sulfur also cycle in ecosystems
Calcium, magnesium, and potassium play important roles in regulating cellular processes and in transmitting signals between cells. Like phosphorus, these macronutrients are derived primarily from rocks and decomposed vegetation. All three can be dissolved in water as positively charged ions: Ca2+, Mg2+, and K+. None is present in a gaseous phase, but all can be deposited from the air in small amounts as dust.
Because of their positive charges, calcium, magnesium, and potassium ions are attracted to the negative charges present on the surfaces of most soil particles. Calcium and magnesium occur in high concentrations in limestone and marble. Because Ca2+ and Mg2+ are strongly attracted to soil particles, they are abundant in many soils overlying these types of rock. In contrast, K++ is only weakly attracted to soil particles and is therefore more susceptible to being leached away by water moving through the soil. Leaching of potassium can lead to potassium-
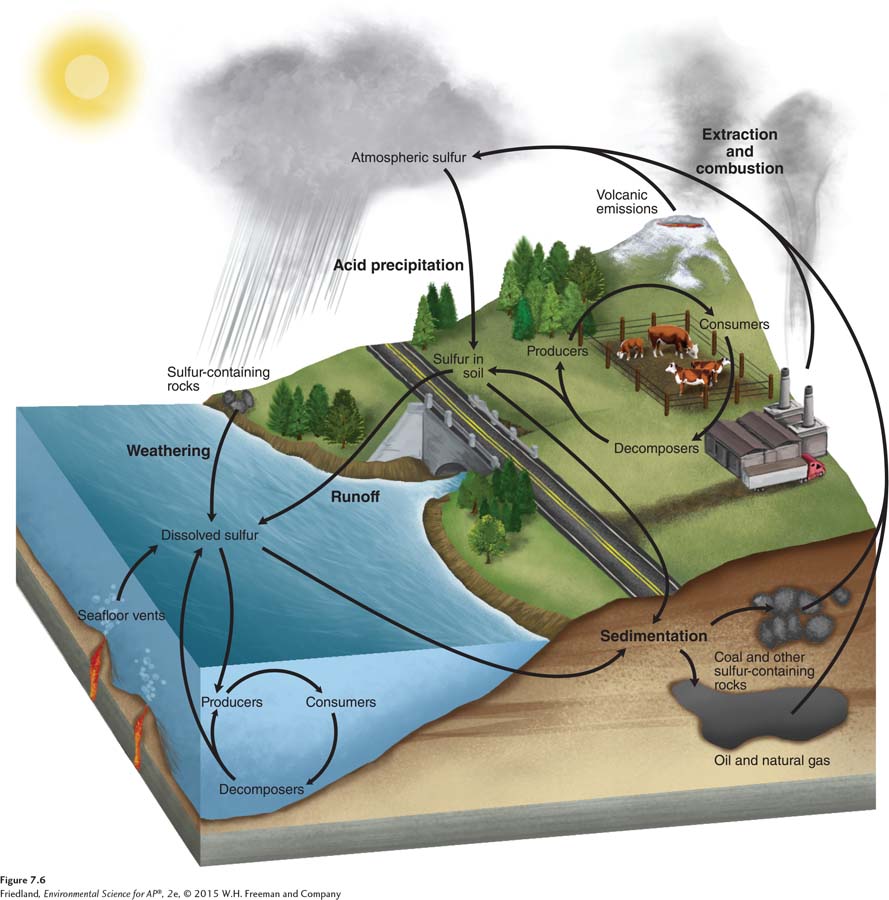
Sulfur cycle The movement of sulfur around the biosphere.
The final macronutrient, sulfur, is a component of proteins and plays an important role in allowing organisms to use oxygen. The sulfur cycle, which is the movement of sulfur around the biosphere, is shown in FIGURE 7.6. Most sulfur exists in rocks and is released into soils and water as the rocks weather over time. Producers absorb sulfur through their roots in the form of sulfate ions (SO42–