Unit 7 AP® Environmental Science Practice Exam
Section 1: Multiple-
Choose the best answer for questions 1–
Question 106.1
1. The owner of a rural home poured several liters of concentrated bleach into a sink drain connected to a buried septic system. Which of the following is the owner most likely to notice after a few weeks?
Settled sludge will be more sterile and dispersed into the leach field.
Sludge has started to accumulate in the leach field.
Sludge has started to accumulate faster in the septic tank.
There is a lower abundance of pathogens in groundwater.
The septage released from the septic tank is cleaner and safer for the environment.
Question 106.2
2. Which of the following sources of mercury pollution may harm humans?
Mercury-
based compounds that are absorbed by marine plankton Inorganic or synthetic mercury-
based compounds Wetlands with mercury concentrations below the legal limit set by the EPA
I only
II only
I and II
II and III
I, II, and III
Question 106.3
3. To remediate an oil spill in the ocean, the use of dispersants
is preferred over the use of hot water sprayers.
can prevent oil from reaching the shorelines.
removes the oil from the ocean.
is less environmentally friendly than the use of oil-
consuming, genetically modified bacteria. is an effective way to slow the damage of oil plumes.
Question 106.4
4. Although thermal and sediment pollution have different sources, they both can
lead to respiratory problems in aquatic animals.
contaminate waterways with heavy metals.
decrease the transparency of water.
lower the temperature of water.
harm fish in the open ocean.
Question 106.5
5. Which of the following is NOT regulated by EPA Clean Water Act or Safe Drinking Water Act?
Levels of arsenic in drinking water
Septage disposal from sewage treatment plants
Nonpoint sources of oil pollution
Levels of giardia in headwater streams
Inorganic chemicals in groundwater
Question 106.6
6. Which of the following groups consists entirely of secondary pollutants?
Carbon dioxide, methane, lead
Particulate matter, nitrogen oxide, nitrogen dioxide
Nitrogen dioxide, sulfur dioxide, ozone
VOC, mercury, carbon dioxide
Nitrate, ozone, sulfate
Question 106.7
7. ___________ is NOT likely to lead to the formation of chemical smog.
Primary production
Combustion of gasoline
Industrial release of CO2
A thermal inversion
Intense sunlight
Question 106.8
8. Sulfur dioxide emissions can be reduced by
selling sulfur emission credits.
increasing the temperature at which coal is combusted.
installing catalytic converters in cars.
installing fabric filters in smokestacks.
using electrostatic precipitators.
Question 9 refers to the following equations:
i + O2→ O3 + UV-
ii + 2O + O2 → iv
Question 106.9
9. Roman numerals i, ii, iii, and iv refer to which of the following compounds?
O, O2, chlorofluorocarbon, 2O2
O, 2O, UV-
C, O + O3 O, O2, energy, O3
Chlorofluorocarbon, O2, chlorofluorocarbon, O3
O, O2 chlorofluorocarbon, O + O3
Question 106.10
10.Which of the following is NOT likely to reduce the risk of sick building syndrome?
Replacing asbestos insulation with more modern insulating material
Installing energy efficient windows
Using hardwood flooring instead of carpeting
Installing carbon monoxide detectors
Using paint with low amounts of volatile organic carbon
Question 106.11
11.Which of the following factors is most likely to decrease the total amount of noncompostable municipal solid waste?
Packaging products in recyclable material
Increasing production of reusable materials
Lowering the cost of disposable products
Manufacturing products that are less durable
Decreasing the production of composite materials
Question 106.12
12.12. Open-
the recycling of a product into the same product.
the recycling of a product into a product that will enter a different waste stream.
the recycling of a product into compost.
a process where only part of the waste is ultimately recycled.
a process where one product is recycled into a different product.
Question 106.13
13.Which factor is least likely to influence the placement of a landfill?
NIMBY politics
Distance of landfill from homes
Local hydrology
Major type of consumer waste
NPP of habitat
Question 14 refers to the following table on the costs associated with landfills and incinerators
| Landfill | Incinerator | ||
| Transporting material to landfill | $10/ton | Transporting material to incinerator | $10/ton |
| Sorting waste | $40/ton | Sorting waste | $40/ton |
| Compacting waste | $30/ton | Burning waste | $160/ton |
| Dumping waste | $10/ton | Removing ash | $30/ton |
Question 106.14
14.Assume an incinerator can produce 500 kWh of energy by incinerating 1 ton of waste, and can sell a single kWh for $0.15. To become economically beneficial, how much more waste would an incinerator have to collect and burn relative to a landfill?
1 ton
1.5 tons
2 tons
4 tons
6 tons
Question 106.15
15.The Comprehensive Environmental Response, Compensation, and Liability Act is referred to as “Superfund” because it
collects taxes from chemical and petroleum industries to fund the cleanup of abandoned waste sites where responsible parties cannot be established.
collects taxes from chemical and petroleum industries to regulate the proper disposal of hazardous waste by small, local businesses.
provides a fund to help both large and small industries to train workers properly on methods of disposing hazardous substances.
provides a fund to help industries design environmental responses to emergencies involving the accidental release of hazardous waste.
distributes money from varying government agencies to chemical and petroleum industries.
Question 106.16
16.Which of the following is an example of an integrated waste management strategy?
Integrating the waste management goals of several small townships into a regional waste management plan.
Developing a nationwide program to handle hazardous waste materials.
Training waste management workers on how to sort recyclables from nonrecyclables.
Simultaneously developing several waste management strategies to reduce the amount of waste that must be incinerated or placed in landfills.
Collecting state taxes to fund the development of more landfills.
Question 106.17
17.HIV, H1N1, and mad cow disease are all similar in that
they can be sexually transmitted.
they have been largely eradicated from developed nations.
they originated from an animal other than humans.
humans infected with these diseases cannot be cured.
they are all coronaviruses.
Questions 18 and 19 refer to the following graph:
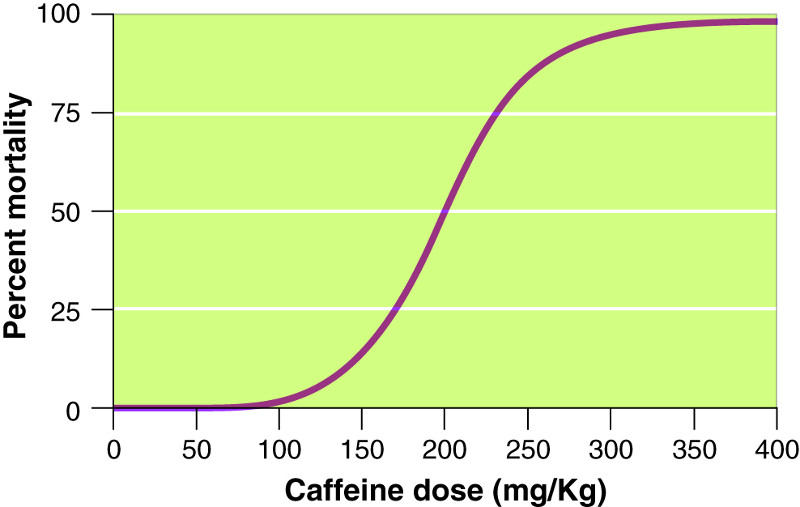
Question 106.18
18.Given the mortality curve in the figure for a 0.5-
10 mg
20 mg
40 mg
100 mg
200 mg
Question 106.19
19.What would be the safe dosage of caffeine for a human?
0.1
0.2
0.3
0.5
0.6
Question 106.20
20.Which factor is the most difficult to predict when quantifying the risk of human exposure to a given contaminant?
Route of contaminant exposure
Solubility of a contaminant in water
Synergistic interactions with other contaminants
Biomagnification of a contaminant
Persistence of a contaminant in water
Section 2: Free-
Write your answer to each part clearly. Support your answers with relevant information and examples. Where calculations are required, show your work.
Question 106.21
1. Concentrated Animal Feeding Operations (CAFOs) house thousands of cows that are concentrated in close living quarters. Manure generated at CAFOs is often washed into massive manure lagoons where it decomposes anaerobically until the manure is sprayed over fields as fertilizer. This practice has several problematic consequences. First, the anaerobic decomposition of manure releases sulfur gases and methane. Second, manure from lagoons can potentially leak into surrounding soils, streams, and lakes. Third, to make room for additional manure in the lagoons, more manure is often sprayed on agricultural fields than is needed for plant growth.
Explain two reasons why the release of sulfur gases poses an environmental and health risk. (2 points)
Name two reasons the leakage of manure from lagoons or the overspraying of manure on agricultural fields poses environmental risks for nearby soils, streams, and lakes. (2 points)
Suggest a retrospective study that could be conducted to determine the effect of manure lagoons on nearby residents. (2 points)
List two possible ways to determine if there is leakage in the soil surrounding a manure lagoon. (2 points)
Which two legislative acts require remediation of groundwater and stream water in cases where a manure lagoon leak occurs? (2 points)
Question 106.22
2. During the first half of the twentieth century, residents in Los Angeles were allowed to have “backyard incinerators” in which they could burn their trash. Although these incinerators reduced the amount of trash going to a landfill, they also created a substantial amount of air pollution that led to severe smog. Initially, Los Angeles attempted to reduce smog by limiting the use of backyard incinerators to the hours between 4 AM and 7 AM. Backyard incinerators were ultimately banned in 1957.
Describe the chemical process that generates smog, and how backyard incinerators are likely to contribute to that process. (2 points)
How would limiting the incineration of trash to the hours between 4 AM and 7 AM potentially reduce the production of smog? (2 points)
Define a thermal inversion and describe how it affects smog. (2 points)
Much of the ash from backyard incinerators was either used in gardens or disposed of in landfills. What is the environmental risk of this practice? (2 points)
How do modern incinerators control the release of both bottom and fly ash? (2 points)