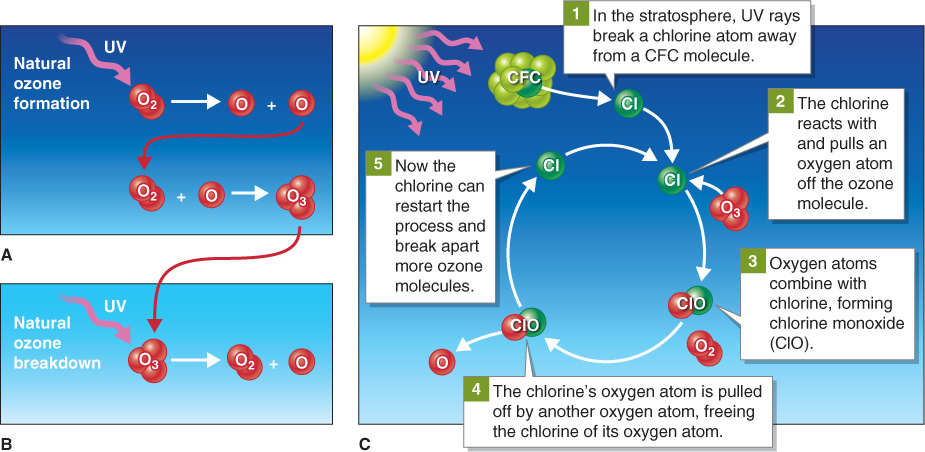
Figure 1.16
Ozone formation and destruction. (A) Ozone forms naturally when ultraviolet (UV) radiation from the Sun breaks apart an oxygen molecule (O2) into two atoms of oxygen (O). One of these atoms combines with a different oxygen molecule, forming ozone (O3). (B) Ozone breaks down naturally when UV radiation breaks ozone molecules back down to oxygen. (C) Ultraviolet radiation breaks CFCs apart into separate atoms of chlorine, fluorine, and carbon. Chlorine then reacts with and breaks apart ozone. A single chlorine atom can convert several hundred thousand ozone molecules to oxygen.