6.3 Carbon and Climate
Compare the long-
Earth’s climate system is strongly influenced by greenhouse gases in the atmosphere, particularly water vapor and carbon dioxide (see Table 1.2). Carbon dioxide has a particularly important influence today because human activities emit more of it, and its anthropogenic emissions are growing faster than any other greenhouse gas. The remainder of this chapter will focus on the role of carbon and carbon dioxide in changing Earth’s climate.
Carbon atoms move among Earth’s physical systems through the carbon cycle. When a covalent bond forms between a carbon atom and two oxygen atoms, a carbon dioxide molecule (CO2) is formed. When this bond is broken, the carbon atom is freed from the oxygen atom. In this section, we will consider both carbon atoms and carbon dioxide molecules as they move through the carbon cycle.
carbon cycle
The movement of carbon through Earth’s physical systems.
The carbon cycle can be divided into a long-
The long-
The Long-Term Carbon Cycle
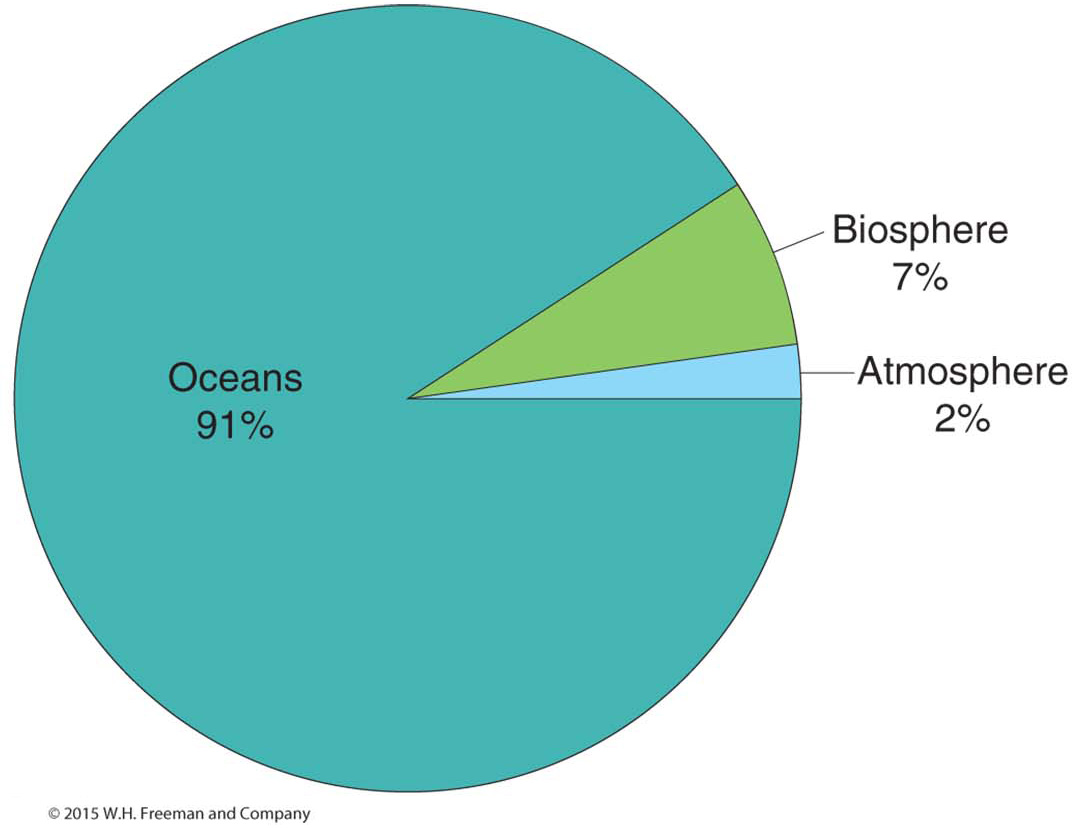
About 99.9% of Earth’s carbon (65,500 billion metric tons) is stored in the lithosphere, where it is bonded with other elements to form different materials, including many types of rocks and fossil fuels (coal, oil, and natural gas). The other 0.1% of Earth’s carbon is found in the oceans, atmosphere, and biosphere. Carbon is moved from the atmosphere, oceans, and biosphere into the lithosphere through weathering and erosion and through the burial and preservation of photosynthetic organisms on land and in the oceans. Carbon leaves the lithosphere through volcanic eruptions and through the burning of fossil fuels.
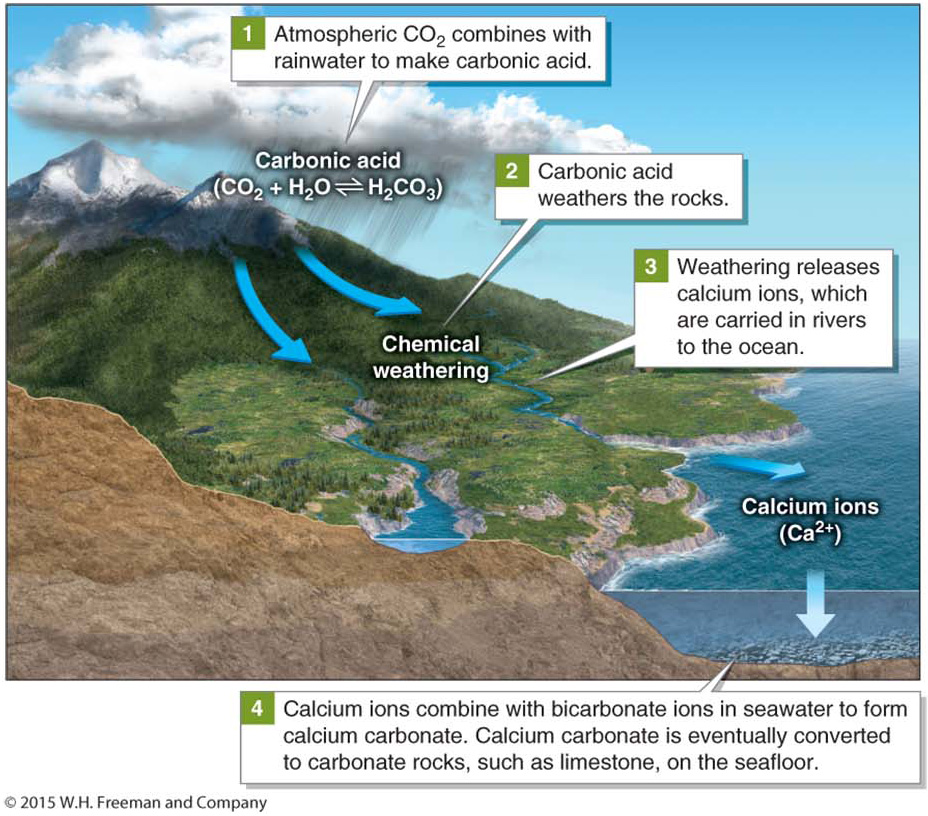
The Role of Weathering and Erosion
Carbon dioxide in the atmosphere combines with rainwater to form a weak acid called carbonic acid (H2CO3). Carbonic acid in rainwater slowly dissolves the rocks over which it flows through the process of chemical weathering. This process releases calcium ions that rivers carry to the oceans. Ions are atoms or molecules with electrical charges that readily react with other particles. Calcium ions combine with bicarbonate ions (HCO3−) in seawater to create chalky white calcium carbonate (CaCO3) sediments, which are similar to the deposits that accumulate on faucets in homes with hard, mineral-
The Role of Photosynthesis
The second way that carbon enters long-
Normally, after these photosynthetic organisms die, they soon decompose. The carbon in their tissues recombines with oxygen in the atmosphere to make carbon dioxide again. Under certain anaerobic (oxygen-
Several hundred million years ago, microscopic photosynthetic marine algae and bacteria (called phytoplankton) and terrestrial forests grew, died, and did not decompose. At that time, Earth was much warmer, and climate favored their preservation. Their remains accumulated and were preserved in marine sediments or in peat wetlands on land. Over millions of years, the preservation of these organisms gradually transferred carbon from the atmosphere and oceans into long-
The Short-Term Carbon Cycle
The short-
Carbon does not take long to move between the biosphere, atmosphere, and oceans. For example, each breath you take moves carbon from the atmosphere to the biosphere (in the form of your body). Whenever an organism dies and decomposes, bacteria recycle the carbon in its body back into the atmosphere and the soil, where it is available to other organisms.
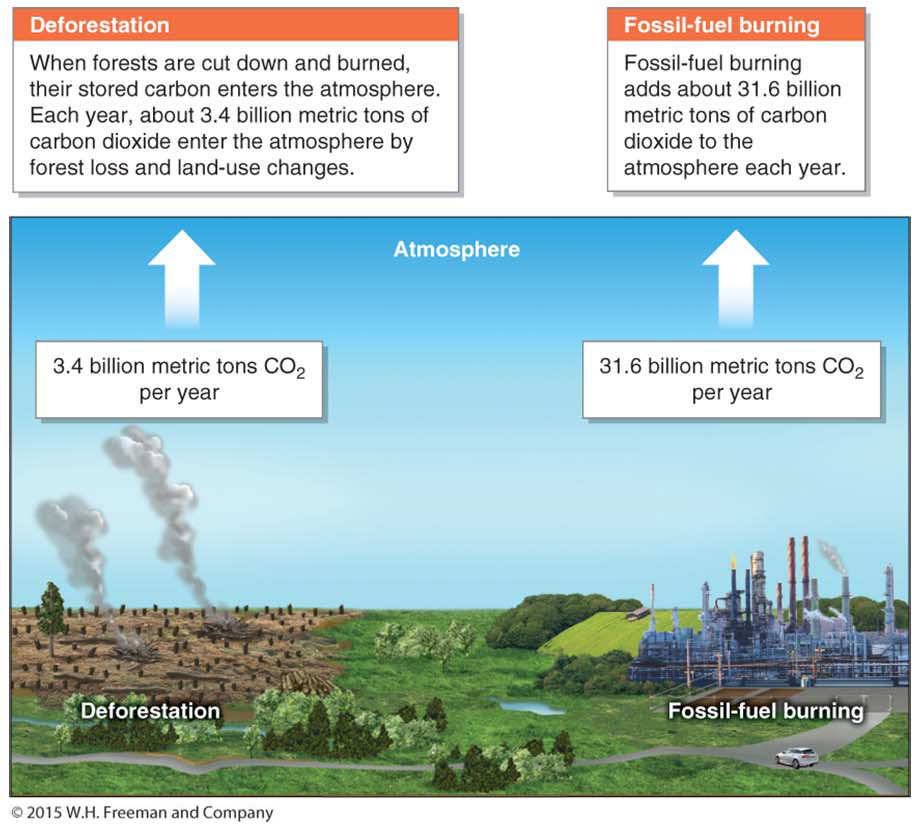
Human Modification of the Carbon Cycle
Once in the lithosphere, carbon cycles back to the atmosphere when volcanoes erupt and when people burn fossil fuels for energy. People have greatly accelerated the transfer of carbon from the lithosphere to the atmosphere by burning fossil fuels. Before the Industrial Revolution, when people first began burning large amounts of fossil fuels, there was an equilibrium between carbon entering the atmosphere through volcanic eruptions and carbon leaving the atmosphere through chemical weathering and sedimentation on the ocean floor (Figure 6.12).
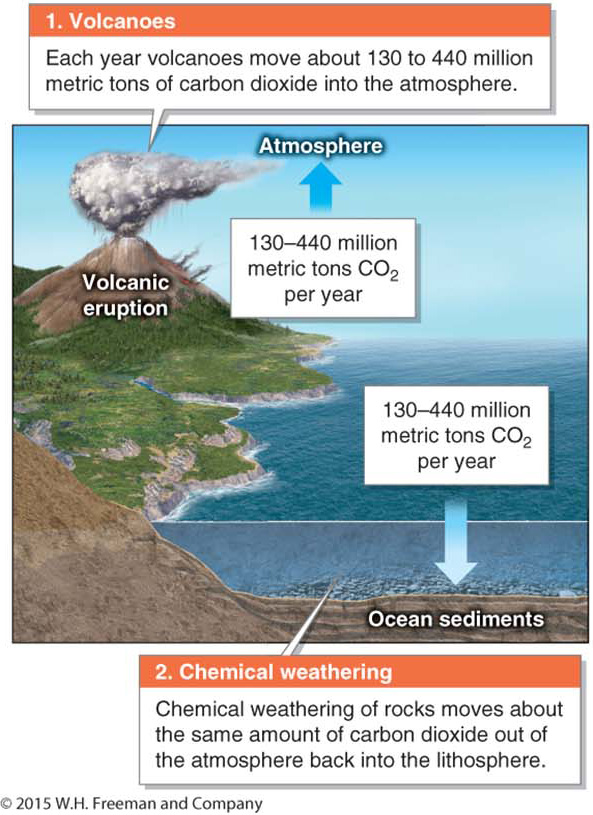
Now people move about 100 to 300 times more CO2 into the atmosphere than all the world’s volcanoes combined. By burning fossil fuels, people are effectively transferring carbon from long-