5.5 Changes in the Brain Over Time
People sometimes speak of the “wiring” of the nervous system, or even of its “hardwiring.” This metaphor is useful for some purposes, but it has its limits. The nervous system is not hardwired like a computer or other human-made device. Neurons are soft, pliable living cells. They can change their sizes, shapes, excitabilities, and patterns of connections in ways that help adapt their possessors to life’s ever-changing circumstances. Every day you grow millions of new synapses and lose millions of others, and at least some of that change depends on the unique experiences you had that day. Furthermore, the brain not only changes during the span of an individual’s life; it has also changed over the course of evolution.
If You Use It, It Will Grow
Like muscles, regions of the brain tend to grow when used and to atrophy when not used. Here is some of the evidence for that statement.
Effects of Deprived and Enriched Environments on the Brain
33
What brain changes have been observed in rats and mice caged in enriched environments?

Early evidence that experience can change the structure of the brain arose from experiments, conducted in the 1960s and later, in which rats were housed in either enriched or deprived environments (Greenough & Black, 1992; Rosenzweig et al., 1972). The enriched environments were large cages in which rats lived with others and had many objects to explore. (“Enriched” is a relative term. These environments were enriched compared to the typical barren cages of laboratory rats but not compared to, say, a garbage dump, where wild rats might live.) The deprived environments were small cages in which each rat lived alone and had no objects except food and a water bottle. After weeks in these environments, the brains of the two groups showed many differences. The brains of the enriched group had thicker cerebral cortexes, larger cortical neurons, more acetylcholine (a prominent neurotransmitter in the cortex), more synapses per neuron, and thicker, more fully developed synapses than did those of the deprived group. These changes occurred even when rats were not placed in the differing environments until well into adulthood. Correlated with these brain differences were marked increases in learning ability in the enriched-environment animals compared to their deprived-environment counterparts.
187
The researchers who performed these early experiments assumed that the brain growth they observed must derive solely from modifications of existing neurons and possibly the addition of new glial cells (the nonneural cells in the brain that provide structural and nutritional support to neurons). As we mentioned earlier, it was believed then that the mammalian brain is incapable of producing new neurons after birth. In the late 1990s, however, researchers using new techniques found ample evidence that new neurons are constantly being generated in some parts of the brain, including the adult human brain (Eriksson et al., 1998).
Generation of new neurons is most apparent in the hippocampus, a structure known to be involved in learning and memory. New hippocampal neurons are generated more rapidly in rats and mice housed in enriched environments than in those housed in deprived environments (Brown et al., 2003; Prickaerts et al., 2004). These new neurons develop functional synapses with already-existing neurons in the hippocampus and appear to contribute significantly to the animals’ capacities for learning and memory (Ge et al., 2007; Toni et al., 2007). Other research shows that many areas of the brain, not just the hippocampus, generate new neurons in response to brain injury (Ming & Song, 2005). These new neurons may well play a role in the gradual recovery of behavioral functions that can occur after brain injury.
Restructuring of the Cortex During Skill Development
34
What evidence shows that practice at a skill alters neural connections so that more neurons become devoted to that skill?
As an animal or person develops skill at a task, ever more neurons in the brain are recruited into the performance of that skill. In one of the first clear demonstrations of this phenomenon, Gregg Recanzone and his colleagues (1992) trained monkeys to discriminate between subtly different rates of vibration applied to a particular patch of skin on one finger. The monkeys received banana pellets for making a certain response each time the vibration rate increased even slightly above 20 cycles per second. Other, “untrained” monkeys received the same vibrations to the skin but were not required to discriminate among them for a food reward. Subsequently, the researchers mapped the somatosensory area of the cortex of all the monkeys by touching points on the skin with a thin probe while recording the activity of cortical neurons. They found that in the trained monkeys the area of the cortex that received input from the “trained” spot of skin was, on average, two to three times larger than the equivalent area in untrained monkeys. Apparently, the brain reorganization resulted not from the skin stimulation per se, but rather from the monkeys’ use of that stimulation to guide their behavior.
188

Subsequently, researchers have found comparable brain changes in visual or auditory sensory areas when animals are trained to discriminate among subtly different sights or sounds (Bakin et al., 1996; Zohary et al., 1994). Research using PET and fMRI neuroimaging has shown that effects like these occur for people, too (Pascual-Leone et al., 2005). In one such study with stringed-instrument players (six violinists, two cellists, and a guitarist), unusually large areas of the somatosensory cortex responded to stimulation of the fingers of the left hand—the same fingers that the musicians had used for years in fingering the strings of their instruments (Elbert et al., 1995).
Some of the most dramatic evidence of the brain’s ability to restructure itself comes from studies of blind people. In sighted people, the whole occipital lobe of the cortex is used for analyzing visual input. Neuroimaging studies have shown that in blind people the occipital lobe becomes devoted to various other purposes, which help them to compensate for their blindness. For example, regions of the occipital lobe that in sighted people are involved in the visual analysis of three-dimensional space become devoted, in the blind, to the task of identifying the locations from which sounds are coming (Gougoux et al., 2005). In Braille readers, large parts of the occipital cortex become devoted to the task of analyzing the tactile input from the fingers in the finally graded way needed to read Braille (Pascual-Leone et al., 2005). Blind people also commonly develop superior verbal memory to compensate for their inability to look up information easily or to find objects by looking. In one fMRI study, blind and sighted people were given lists of nouns to memorize as their brains were scanned (Amedi et al., 2003). The blind subjects showed marked activation of portions of the occipital cortex during this task, which did not occur in the sighted people, and they also showed superior memory. Moreover, those blind subjects who scored best on the memory test showed the most activity in the occipital cortex.
Alvaro Pascual-Leone and his colleagues (2005) found that at least some of these brain changes began to occur in sighted people who had been blindfolded for just 5 days. When the blindfolds were removed, the changes quickly reversed themselves.
Spatial Learning and Growth of the Hippocampus
35
What evidence, with birds and with humans, indicates that spatial learning can result in growth in the hippocampus?
As described in Chapter 4, some bird species hide seeds in multiple locations and retrieve them in the winter, and these birds generally have larger hippocampi than do related species that do not hide seeds. Researchers working with one of these species, the mountain chickadee, have shown that enlargement of the hippocampus depends at least partly on experience (Clayton, 2001). When caged chickadees are allowed to hide and retrieve seeds, their hippocampi grow, and when they are then prevented from hiding and retrieving seeds for a period of time, their hippocampi shrink again. The hippocampus is involved in many forms of memory, especially memory for spatial locations.
189
A study of London taxi drivers suggests that extensive spatial learning can increase hippocampal size in humans, too. Cab drivers in big cities develop remarkable spatial abilities, and this is especially true of London cabbies, who, to get a license, must go through prolonged training and pass a test of their ability to find the shortest route between any two locations in that large city. Brain scans revealed that the posterior (rear) part of the hippocampus (the part most involved in spatial memory) is significantly larger in London cab drivers than in otherwise similar people who do not drive taxis (Maguire et al., 2000). They also revealed a significant positive correlation between years of cab-driving experience and growth in the hippocampus: In general, the longer a person had been driving a cab, the larger was the posterior hippocampus.
Strengthening of Synapses as a Foundation for Learning
Learning undoubtedly involves many types of changes in the brain. But at the cellular level the type of change that has been most clearly linked to learning is the strengthening of synaptic connections between already existing neurons.
The Hebbian Synapse: Neurons That Fire Together Wire Together
36
How has the discovery of long-term potentiation tended to confirm Hebb’s theory about synaptic strengthening?
Many years ago, the Canadian psychologist Donald Hebb (1949) theorized that some synapses in the brain have the property of growing stronger (more effective) whenever the postsynaptic neuron fires immediately after the presynaptic neuron fires (see Figure 5.34). Through this means, Hebb suggested, neurons could acquire the capacity to respond to input that they previously didn’t respond to. This could provide a basis for classical conditioning and other forms of learning. In the 1970s, Timothy Bliss and Terge Lømo (1973) discovered a phenomenon called long-term potentiation, or LTP, which strongly supports Hebb’s theory.
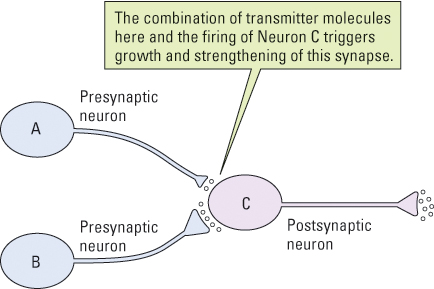
In the laboratory, LTP is produced by artificially stimulating, with a burst of electrical pulses, a bundle of neurons entering a particular region of an animal’s brain. This results in the strengthening of the synapses that those neurons form with postsynaptic neurons so that subsequent weak stimulation of the same bundle elicits a stronger response in the postsynaptic neurons than it would have before. This potentiation (strengthening) is long term: It lasts for hours or even months, depending on the conditions. Subsequent research has shown that, at least in some brain areas, LTP works in the following manner.
Imagine a weak synapse between neuron A and neuron C (depicted in Figure 5.34). When neuron A becomes active, some of the neurotransmitter molecules it releases become bound to conventional, fast-acting receptors on the postsynaptic membrane, where they produce a depolarization that is too slight to play a significant role in triggering action potentials. Other transmitter molecules at the same synapse, however, become bound temporarily to special LTP-inducing receptors on the postsynaptic membrane. If neuron C then fires an action potential (due to input from other neurons, such as B in Figure 5.34), the combination of that firing and the presence of transmitter molecules in the LTP-inducing receptors triggers a series of biochemical events that strengthen the synapse (Bi & Poo, 2001; Byrne, 2008). The presynaptic terminal becomes larger, able to release more transmitter substance than it could before, and the postsynaptic membrane develops more conventional receptor sites than it had before.
As a result of such changes, firing in neuron A produces more depolarization in neuron C than it did before and therefore plays a greater role in triggering action potentials in that cell than it did before. Sets of neurons that behave like this have been found in many parts of the mammalian brain, including various areas of the cerebral cortex, the hippocampus, the cerebellum, and the amygdala—all of which are known to be involved in various kinds of learning (Byrne, 2008).
190
Evidence That Long-Term Potentiation Is a Basis for Learning
37
What evidence shows that long-term potentiation is involved in learning?
Evidence that LTP is actually involved in learning comes from many experiments showing that interference with the brain’s normal capacity for such potentiation interferes with the animal’s ability to learn (Byrne, 2008). In one experiment with mice, for example, a drug that prevents LTP was injected into a portion of the amygdala that is known to be crucial for fear learning. After this treatment, the researchers tried, through classical conditioning, to train the mice to fear a tone that was paired with an electric shock. (As you may recall from Chapter 4, classical conditioning occurs when a neutral stimulus, which does not initially elicit a response, is paired with an unconditioned stimulus, which does elicit a response. After a sufficient number of pairings, the neutral stimulus, now called a conditioned stimulus, elicits a response even in the absence of the unconditioned stimulus.) The LTP-inhibited mice failed to learn such fear. They responded to the shock as did normal mice, but, unlike normal mice, they did not subsequently show fear responses to the tone (Maren, 1999).
Given such evidence that LTP is essential for learning, what would happen if the capacity for LTP were increased above the normal level? Joe Tsien (2000) and his colleagues found a way to answer that question. The postsynaptic receptors that are involved in initiating LTP come in two forms—a strong form, which is highly effective in triggering LTP, and a weak, less effective form. Through genetic engineering, Tsien and his colleagues produced a strain of mice—which they named “Doogie,” after the boy genius of the old television show Doogie Howser, M.D. —that had many more of the strong receptors and fewer of the weak ones than do normal mice. As predicted, brain neurons in the Doogie mice showed more LTP in response to electrical stimulation than did those in the normal, unaltered mice. Remarkably, but just as predicted, the Doogie mice also showed better memory than the unaltered mice in a series of three widely different tests: maze learning, classical conditioning of a fear response, and object recognition (see Figure 5.35). In each case, the altered mice behaved similarly to the unaltered mice during the learning experience but showed significantly better memory when tested a day or more later (Tang et al., 1999; Tsien, 2000). More recently, Tsien and his colleagues showed that such genetic modification can also prevent the decline in memory that normally occurs in aged mice (Cao et al., 2007).

The results of these experiments raise many questions that have not yet been answered. Will similar results be found with other animals and other forms of learning? Could memory in people be improved through methods that increase LTP? If a simple genetic change can improve memory by increasing LTP, then why hasn’t evolution already produced that change through natural selection? Perhaps LTP beyond a certain level, or even memory beyond a certain level, is maladaptive in the conditions of everyday life.
191
The Evolution of the Human Brain
As we’ve seen earlier in this chapter and in the previous section, brains change over the course of an individual’s development and in response to learning. However, the brain has also changed over longer stretches of time. Here, we’ll discuss briefly the evolution of the human brain, particularly how the brain changed over the last 5 to 7 million years, since we last shared a common ancestor with modern chimpanzees and bonobos.
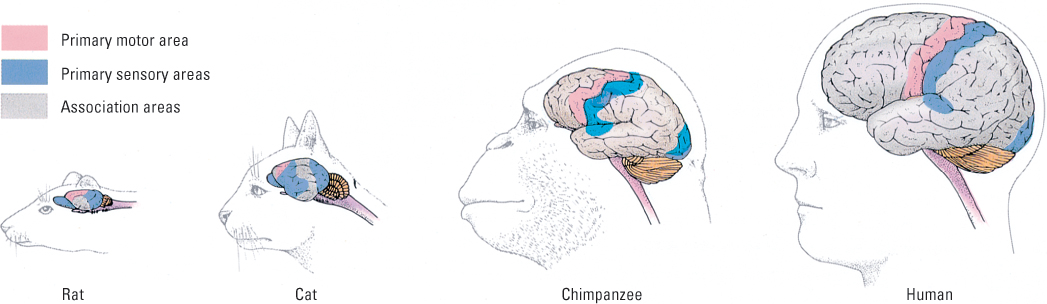
Although most of our discussion to this point has been about human brains, brain organization is very similar in all mammals. What has changed appreciably over evolutionary time, however, is the amount of volume dedicated to the associative areas of the brain relative to the sensory and motor areas. Figure 5.36 shows that the amount of association cortex, relative to the other two categories, increases dramatically as one goes from simpler mammals, such as the rat and the cat, to more complex ones, such as the chimpanzee and the human.
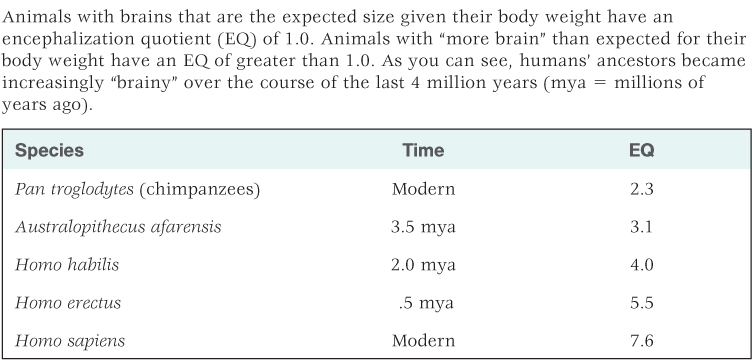
In fact, the most prominent feature of the human brain is its size. In comparison to other mammals, the human brain is much larger than expected in proportion to human body size. Primates in general have large brains relative to their body size, but humans’ brains are especially large. Harry Jerison (1973, 2002) developed a formula for evaluating the expected ratio between brain weight and body weight for animals called the encephalization (en-sěf´-ǝ-lǝ-zā´-shon) quotient, or EQ. Animals with brains that are the expected weight given their body weight have an EQ of 1.0. Animals with smaller brains than expected for their body weight have an EQ of less than 1.0, and animals with larger brains than expected for their body weight have an EQ of greater than 1.0. Using this measure, humans have an EQ of about 7.6, more than triple that of chimpanzees at 2.3 (Jerison, 1973, 2002; Rilling & Insel, 1999). This change in relative brain size has apparently occurred gradually since humans and chimpanzees last shared a common ancestor. Using fossil evidence, EQs can be derived for human ancestors, and these are provided for three such species (Australopithecus afarensis, Homo habilis, and Homo erectus), along with modern chimpanzees and humans, in Table 5.2. As you can see, the EQ for Australopithecus afarensis, an early small-brained species that lived 3 to 4 million years ago, was only slightly greater than that of chimpanzees. From this point on, brain weight relative to body weight increased at a rapid rate.
Estimated encephalization quotients (EQ) for chimpanzees (Pan troglodytes) and four hominid species
Scientists have discovered genetic differences in genes between modern chimpanzees and humans that regulate brain development and are related to differences in brain size and function (Khaitovich et al., 2005; Pollard et al., 2006). Moreover, natural selection has apparently tinkered with brain size quite recently. For instance, one version of a gene related to increased brain size arose as recently as 37,000 years ago (Evans et al., 2005) and another a mere 5,800 years ago (Mekel- Bobrov et al., 2005).
192
Although humans are physically different from chimpanzees and other apes in many ways (their digestive tracts are quite different, for example), the difference that is most notable is in their brains. This is because brains are the eventual source for behavior and cognition—where chimpanzees and humans truly diverge. Chimpanzees and humans have enough in common so we can get a glimpse at how natural selection could have worked on the thinking and behavior of ancient apes to produce the human mind. But although contemporary chimpanzees and humans both use tools, transmit information across generations, and form social coalitions, the gap between the two species in executing these skills is enormous. And the primary organ responsible for this gap is the brain.
SECTION REVIEW
The brain physically changes in response to experience.
Growth and Reorganization
- Rats in enriched environments develop thicker cortexes with larger neurons and more and stronger synaptic interconnections. They also generate new neurons at a faster rate.
- Skill learning causes larger portions of the brain to become involved in performing that particular skill.
- The hippocampus, an area critical to spatial memory, grows as a result of spatial learning (as demonstrated in London taxi drivers).
Long-Term Potentiation (LTP)
- LTP strengthens synaptic connections in ways that mediate learning.
- In line with Hebb’s theory, the coordinated firing of a presynaptic and postsynaptic neuron strengthens the synaptic connections of the first onto the second.
- LTP involves enlargement of axon terminals and generation of new receptor sites on postsynaptic membranes.
Human brains are substantially larger than expected for their body weight, more so than any other species.
- This difference is greatest in the association (“thinking” cortex).
- Gradual changes in relative brain size can be seen in the line that led to modern humans.
- Genes associated with brain size have arisen relatively recently in human evolution.
193