6.2 Reward Mechanisms of the Brain
As we noted in the previous section, motivated behavior involves the pursuit of rewards (also known as incentives, goals, or reinforcers). Let us now look more closely at the concept of reward and at research into how rewards act on the brain to promote and reinforce the behaviors that led to them.
Three Components of Reward: Liking, Wanting, and Reinforcement
6
What are three interrelated components of the concept of reward?
In psychology, the term reward has three interrelated, but in some ways separable, meanings. A reward is something that we like, something that we want, and something that serves as a reinforcer in learning (Berridge & Kringelbach, 2008).

Liking refers to the subjective feeling of pleasure, or satisfaction, that occurs when one receives a reward. We know this feeling from our own experience. We experience pleasure from good food when we are hungry, from water when we are thirsty, from drifting off to sleep when we are tired, and from sexual activity when we are sexually motivated. We also experience pleasure from pay, praise, the company of good friends, play, music, discoveries made through exploration, and our own assessment of a job well done. Most of the things we seek in life bring us pleasure when we obtain them. We have no way to know for sure that other mammals also experience pleasure from the rewards they receive, but their behavior certainly suggests that they do (see Figure 6.2).
201
Wanting refers to the desire to obtain a reward. This is the component of reward that links most clearly to the concept of motivation. To want something is to be motivated to get it. Whereas pleasure occurs when a reward is received, wanting occurs before it is received. Wanting is typically measured by assessing the amount of effort an individual will exert, or the amount of pain the individual will bear, in order to obtain the reward. Usually objects that are wanted are also liked, but, as you will see later, it is possible to separate the two.
Reinforcement refers to the effects that rewards have in promoting learning. As discussed in Chapter 4 (in the section on operant conditioning), animals and people learn to attend to stimuli that signal the availability of rewards, and they learn to make responses that bring rewards in the presence of those stimuli. Somehow, through its effects on the brain, a reward helps to stamp in, or reinforce, the memory of stimuli and actions that occurred just before the reward was received. Such learning helps the individual to become more effective in finding and procuring the same type of reward in the future.
Studies of the brain, to which we now turn, have provided some clues to the mechanisms of each of these three components of reward.
Identification of Reward Neurons in the Brain
1
How did Olds and Milner identify reward pathways in the brain?
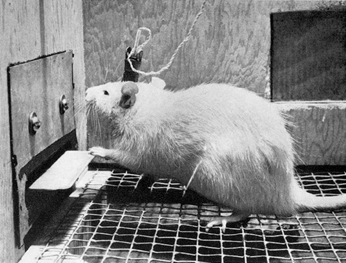
The study of brain mechanisms of reward was initiated in the 1950s, when James Olds and Peter Milner made a remarkable discovery. These researchers observed, by accident at first, that when rats received electrical stimulation through thin wires implanted in certain brain areas, they behaved as if they were trying to get more of that stimulation. For example, if a rat happened to receive the stimulation while exploring a particular corner of the cage, the animal would return repeatedly to that corner. To see if such brain stimulation would serve as a reinforcer for learning, Olds and Milner tested rats in an apparatus in which they could electrically stimulate their own brains by pressing a lever (see Figure 6.3). With electrodes placed in certain brain areas, rats learned very quickly to press the lever and continued to press at high rates, sometimes for many hours without stopping (Olds & Milner, 1954).
8
What is some evidence that the medial forebrain bundle and nucleus accumbens are essential pathways for the effects of a wide variety of rewards?
Subsequent research showed that rats and other animals will work hardest and longest to stimulate a tract in the brain called the medial forebrain bundle. The neurons of this tract that are most crucial for this rewarding effect have their cell bodies in nuclei in the midbrain and synaptic terminals in a large nucleus in the basal ganglia called the nucleus accumbens (see Figure 6.4). The nucleus accumbens itself has connections to large areas of the limbic system and the cerebral cortex, and it is now understood to be a crucial center for the behavioral effects of rewards, in humans as well as in other mammals.
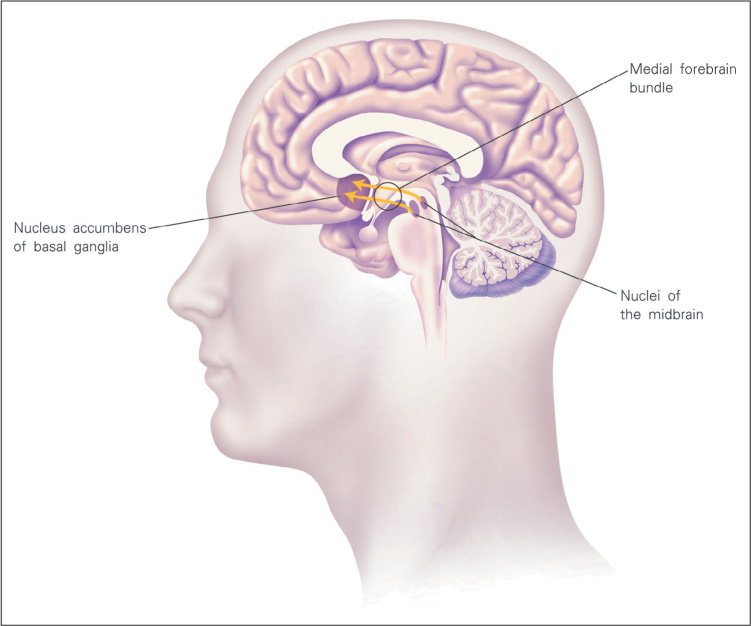
When Olds and Milner inserted electrodes into the medial forebrain bundle, they were tapping artificially, but very effectively, into the brain’s natural reward system. Subsequent research, involving the recording of activity in the brain, has shown that the medial forebrain bundle and the nucleus accumbens become active in all sorts of situations in which an individual receives a reward, whether the reward is food, opportunity to copulate, novel objects to explore, or (in humans) a prize received for winning a game (Breiter et al., 2001; Fiorello et al., 2003; Koob et al., 2012). Moreover, damage to either of these brain structures destroys all sorts of motivated behaviors (Koob et al., 2012). Without a functioning medial forebrain bundle or nucleus accumbens, animals will not work to find or obtain rewards and will die unless they are provided with food and water through a stomach tube.
202
Separating the “Liking” and “Wanting” Systems
9
What is some evidence that different neurotransmitters in the nucleus accumbens are involved in the “wanting” and “liking” components of reward?
Many of the neurons of the medial forebrain bundle that terminate in the nucleus accumbens release dopamine [dōp′-ǝ-mēn’] as their neurotransmitter. This release appears to be essential for the “wanting” component of reward, but not for the “liking” component. Animals that have been well trained to press a lever for some reward, such as food, show a release of dopamine into the nucleus accumbens just before they begin to press the lever, but not after they receive the reward (Phillips et al., 2003). This pattern is consistent with the idea that dopamine helps motivate the animal to obtain the reward (promotes “wanting”) but is not essential for the pleasure received from obtaining the reward. Other research shows that the larger the expected reward, the greater the degree of dopamine release in the nucleus accumbens (Roesch et al., 2007).
More direct evidence that dopamine is involved in “wanting” but not “liking” comes from studies in which rats are treated with drugs that block the effect of dopamine in the nucleus accumbens. These animals continue to consume foods, copulate with sexual partners, and explore novel stimuli that are immediately present. They also continue to exhibit the facial “liking” expression (depicted in Figure 6.2) when they taste a sugar solution. However, they do not continue to seek out or work for rewards that are not immediately present (Berridge & Robinson, 2003; López & Ettenberg, 2002; Zhang et al., 2003). Their behavior suggests that they continue to enjoy the consumption of rewards but are no longer concerned with (no longer behave as if they want) rewards that are absent. Conversely, drugs that increase the activity of dopamine in the nucleus accumbens increase the rate at which rats and other animals will work for food, but do not increase the facial “liking” response to sucrose or the animal’s consumption of food that is immediately available (Berridge & Robinson, 2003).
203
If dopamine is responsible for the “wanting” component of reward, what is responsible for the “liking” component? Some of the neurons of the medial forebrain bundle that terminate in the nucleus accumbens release not dopamine but a different transmitter, one that is in the endorphin [ěn-dȯr′-fǝn] family. The term endorphin is short for “endogenous morphine-like substance.” (Endogenous means “created within the body.”) Endorphins are chemicals created within the body that have effects similar to those of morphine and other opiate drugs such as opium and heroin; they are best known for their role in inhibiting the sense of pain. A number of experiments suggest, however, that endorphins released into the nucleus accumbens are also crucial for the immediate pleasure experienced when rewards are received or consumed. When drugs that activate endorphin receptors are injected into the nucleus accumbens, they increase the facial “liking” reaction to sucrose (Smith & Berridge, 2007) and also increase the amount of immediately present food that an animal will eat (Zhang & Kelley, 2000). In humans, drugs that decrease the effectiveness of endorphins have been reported to decrease people’s perceived enjoyment of food and other rewards (Yeomans & Gray, 1996).
Role of Dopamine in Reinforcement for Learning
10
What evidence suggests that dopamine is crucial to the capacity of rewards to promote new learning—that is, to serve as reinforcers?
The learning component of reward is closely related to the “wanting” component. Animals learn that certain cues signal the availability of a reward, and those cues prompt the animal to search for or work for the reward, which is the behavioral indicator of “wanting.” The release of dopamine into the nucleus accumbens appears to be crucial not just for motivating animals to work for rewards, but also for their ability to learn to use cues to predict when and where rewards are available. One line of evidence for this is the observation that dopamine release promotes long-term potentiation (LTP) of neural connections within the nucleus accumbens (Reynolds et al., 2001). As discussed in Chapter 5, LTP is believed to be part of the cellular basis for learning throughout the brain.
Other evidence comes from studies in which the amount of dopamine released into the nucleus accumbens is directly measured as animals anticipate and receive rewards (Day et al., 2007; Schultz, 1998). If food is presented to a hungry monkey or rat occasionally, at unpredictable times, a burst of dopamine is released into the nucleus accumbens each time food is presented. If the situation is then changed so that a signal light comes on a few seconds prior to each presentation of food, the animal soon learns to anticipate food each time the light comes on. In this new situation, after several trials, a burst of dopamine release occurs when the light comes on, but not when food is presented. The animal eats and apparently enjoys the food, but no dopamine release accompanies that behavior.
This pattern of dopamine release is consistent with the idea that dopamine is involved in new learning (Schultz, 1998). When a reward is unexpected, dopamine release immediately after the reward helps to reinforce an association between the reward and any stimulus or response that happened to precede it. When the cues and responses leading to a reward have already been well learned, however, there is no need for further reinforcement of that learning, and dopamine release in response to the reward (the food) ceases. Dopamine release now occurs in response to the signal preceding reward (the light) because now the animal’s interest lies in learning how to predict when the signal will appear or how to make it appear.
Hijacking the Brain’s Reward System
11
How does an understanding of the brain’s reward system help us to understand drug addiction and compulsive gambling?
When Olds and Milner delivered electrical impulses into the medial forebrain bundle in rats, they were short-circuiting the rats’ natural reward systems. When people get “hooked” on drugs or on gambling, they are doing essentially that same thing with their own brains.
204
Drug Addiction
Cocaine, amphetamine, heroin, opium, and other often-abused drugs exert their euphoric and habit-producing effects through action on the brain’s reward pathways (Koob et al., 2012). In various ways, these drugs mimic or promote the effects of dopamine and endorphins in the nucleus accumbens.

Rats fitted with mechanisms for pumping drugs into their bloodstreams will self-administer cocaine and other such drugs, and become addicted, but will stop self-administering the drugs if the nucleus accumbens is destroyed or chemically blocked (Wise, 1996). Rats will work as hard to administer tiny amounts of cocaine or amphetamine through a cannula (tiny tube) directly into the nucleus accumbens as they will to administer much larger amounts into the blood stream (Hoebel et al., 1983; Wood & Emmett-Oglesby, 1989). These findings are consistent with other evidence that the nucleus accumbens is a key area where drugs act to produce their addictive effects.
Our understanding of the brain’s reward mechanisms gives us a clue as to why such drugs are addictive. Not only do they produce an immediate sense of euphoria, but even more significant for the problem of addiction, they strongly activate the dopamine-receiving neurons in the nucleus accumbens that are responsible for promoting reward-based learning. Normal rewards, such as food, activate these neurons only when the reward is unexpected; but cocaine and other addictive drugs, through their direct chemical effects, activate these neurons every time the drug is taken. The result may be a sort of super learning (Hyman et al., 2006). With each dose of the drug, the dopamine response acts to reinforce, once again, associations between any cues that are present in the environment and the feelings and behaviors of wanting and taking the drug. The result is the buildup of an extraordinarily strong craving and habit, which are triggered whenever cues that have been present during past drug-taking are present.
It has often been observed that drug addicts gradually lose their “liking” of the drug (experience less pleasure) over time, even while their “wanting” of the drug increases (Kelley & Berridge, 2002). The loss in liking occurs, presumably, because of drug-induced changes in the brain that reduce the endorphin-mediated pleasure response. However, because the dopamine response is not reduced, the learned drug craving and habit continue to grow stronger with each dose (Kelley & Berridge, 2002; Nestler & Malenka, 2004). The craving itself, rather than any expected pleasure, becomes the main reason for taking the drug. Drug taking becomes a compulsion rather than something that one freely chooses to do for pleasure. (Another reason why drug addicts continue to take drugs, having to do with conditioned counteractive responses, was discussed in Chapter 4.)
A Brain-Based Theory of Compulsive Gambling
In North America, somewhere between 1 and 2 percent of adults suffer from a compulsive, pathological drive to gamble (Grant et al., 2004), a drive that persists even though it may leave the person and his or her family in financial ruins. Compulsive gambling is in some ways similar to drug addiction (Holden, 2001). Gamblers claim to feel a euphoric high when they are gaming and winning, and to experience withdrawal symptoms—such as sweating, restlessness, and sleeplessness—when they try to abstain. Every cue in the environment that has been previously associated with gambling elicits in them a strong, often irresistible urge to gamble. Our understanding of the brain’s reward mechanisms gives us some clues about the origins of this compulsion.

Brain imaging studies with healthy human subjects (using the fMRI technique, discussed in Chapter 5) have revealed that games of chance with monetary rewards are powerful activators of the nucleus accumbens and other structures known to be part of the brain’s reward system (Breiter et al., 2001; Knutson et al., 2001). Because the payoff is never predictable, every instance of payoff results in a new burst of dopamine release in the nucleus accumbens, no matter how many times the person plays. Thus, gambling, like drug taking, overrides the brain’s dopamine-conserving mechanism—the mechanism that shuts off the dopamine response once the reward has become predictable.
205
Consciously, the person may know that the game pays off in a way that is unpredictable and uninfluenced by anything that he or she does, but the brain’s primitive reward system nevertheless behaves as if it is constantly trying to learn how to predict and produce the reward. The repeated reinforcement, by dopamine, of associations between payoffs and the cues and behaviors that precede each payoff results in the buildup of an abnormally strong habit.
People who, for genetic reasons, have high levels of dopamine receptors in their brains have an unusually high susceptibility to compulsive gambling (Sabbatini da Silva Lobo et al., 2007). The same is true for people who, because of Parkinson’s disease or other disorders, are taking drugs that increase the potency of dopamine transmission in the brain (Giladi et al., 2007; Quickfall & Suchowersky, 2007). These findings are consistent with the theory that compulsive gambling is reinforced by the dopamine response to un-predicted rewards.
SECTION REVIEW
Brain reward systems mediate three aspects of reward: wanting, liking, and reinforcement.
Neurochemistry of Reward
- The medial forebrain bundle and nucleus accumbens contain essential pathways of the brain’s reward system. Animals will work for electrical stimulation in these areas.
- The release of dopamine into the nucleus accumbens is associated with wanting; the release of endorphins into this area is associated with liking.
- The release of dopamine into the nucleus accumbens is also crucial to reinforcement; it promotes learning how to predict and obtain a given reward.
Drug Addiction and Compulsive Gambling
- Addictive drugs cause dopamine release into the nucleus accumbens each time they are taken, which may cause super-reinforcement of cues and actions associated with obtaining the drug; hence, addiction.
- Because of the unpredictability of rewards in gambling, each reward may stimulate release of dopamine into the nucleus accumbens, resulting in super-reinforcement of cues and actions associated with gambling.