6.3 Hunger: An Example of a Regulatory Drive
It is no accident that eating is one of life’s great pleasures. Throughout our evolutionary history, the difficulty of finding adequate food was one of the major barriers, if not the major barrier, to survival. As a result, natural selection built into us (and other animals) powerful, robust hunger mechanisms that lead us to search for food, to eat when food is available, and to experience pleasure when we eat. Natural selection also built into us satiety mechanisms, which tend to keep us from overeating and becoming obese. But the satiety mechanisms are not as robust as the hunger mechanisms. In our evolutionary history, food scarcity was a much bigger problem than overabundance. Far more people died of starvation than of obesity, and this is still true in many parts of the world today. Things are different, however, for those of us who live in postindustrial parts of today’s world. For us (and especially for those living in North America), obesity has become a major health problem. In what follows, we will examine some of the mechanisms that control appetite and then look at the modern problem of obesity.
206
Neural and Hormonal Control of Appetite
12
What is meant by feedback control, and how does the arcuate nucleus of the hypothalamus serve as a control center for appetite?
The purpose of hunger and satiety is to regulate the amount of food materials in the body at an appropriate level for survival and well-being. Any regulatory system, whether manmade or organic, makes use of feedback control. The substance or quality being regulated feeds back upon the controlling device and inhibits the production of more of that substance or quality when an appropriate level is reached. A home thermostat, which controls the operation of the heating system, is a good example. The thermostat is sensitive to temperature. When the temperature is low, a switch closes in the thermostat, which turns on the furnace, which provides heat. When the temperature rises above the set level, the switch opens, and the furnace turns off. Thus heat, produced by the furnace, feeds back onto the thermostat to shut off the source of heat when enough of it is present.
The mammalian brain regulates food intake in a manner that is a bit like the operation of a home thermostat, but far more complicated. Sets of neurons in the brain’s hypothalamus raise or lower the animal’s drive to eat, and these neurons are themselves regulated by the body’s deficit or surfeit of food materials. We might think of these neurons as the brain’s “food-o-stat.” When food materials are relatively low in the body, the food-o-stat cranks appetite up, which motivates the animal to consume more food. When food materials are plentiful in the body, various indicators of that plenitude feedback upon the food-o-stat and turn appetite off, or at least down a bit.
A Nucleus in the Hypothalamus Serves as an Appetite-Control Center
The neurons that constitute the food-o-stat exist in several closely interconnected portions of the hypothalamus, but are most concentrated in the arcuate [ark′-yū-ǝt] nucleus, which lies in the center of the lowest portion of the hypothalamus, very close to the pituitary gland (Berthoud & Morrison, 2008). This tiny brain area has been described as the “master control center” for appetite and weight regulation (Marx, 2003). It contains two classes of neurons that have opposite effects on appetite. One class, the appetite-stimulating neurons, connect to various parts of the brain and promote all the effects that are associated with increased hunger, including craving for food, increased attention to food-related cues, increased exploration in search of food, and heightened enjoyment of the taste of food. The other class, the appetite-suppressing neurons, have effects on various parts of the brain that are opposite to those of the appetite-stimulating neurons.
Both of these classes of arcuate neurons exert their effects on other brain areas through the release of slow-acting neurotransmitters, which have the capacity to alter neural activity for long periods of time—in this case for periods ranging from minutes to several hours. One of the neurotransmitters released by appetite-stimulating neurons is neuropeptide Y (abbreviated NPY), which is the most potent appetite stimulator yet discovered. When injected into any of various regions in the hypothalamus, this chemical causes a previously sated animal to eat voraciously (Stanley & Gillard, 1994). The neurons of the arcuate nucleus are themselves acted upon by many different inputs that, in one way or another, reflect the need or lack of need for food.
Many Internal Signals Contribute to Short-Term Regulation of Appetite
Eating a large meal produces a number of physiological changes in the body. Among these are slightly elevated body temperature (resulting from a heightened rate of metabolism), increased blood level of glucose (a simple sugar molecule derived from the breakdown of carbohydrate foods), distention of the stomach and intestines (resulting from food inside those structures), and the release of certain hormones produced by endocrine cells in the stomach and intestines. There is evidence that all these changes can either directly or indirectly incite neurons in the arcuate nucleus and nearby areas of the hypothalamus to activate hunger-suppressing neurons and inhibit hunger-stimulating neurons (Berthoud & Morrison, 2008; Korner & Leibel, 2003). When all these effects are operating properly, the result is a decline in appetite for several hours following ingestion of a meal.
207
One appetite-suppressing hormone that has received considerable attention is peptide YY3-36 (abbreviated PYY), which is produced by special endocrine cells in the large intestine. Food entering the intestines after a meal stimulates secretion of PYY into the bloodstream. In humans, blood levels of the hormone begin to increase 15 minutes after a meal is eaten, peak at about 60 minutes, and remain elevated for as long as 6 hours after a large meal (Batterham et al., 2003). Research with rodents shows that one of the target tissues of PYY is the arcuate nucleus, where the hormone excites appetite-suppressing neurons and inhibits appetite-stimulating neurons (Marx, 2003).
13
What is the evidence that the hormone PYY helps reduce appetite after a meal and that underproduction of PYY may contribute to obesity?
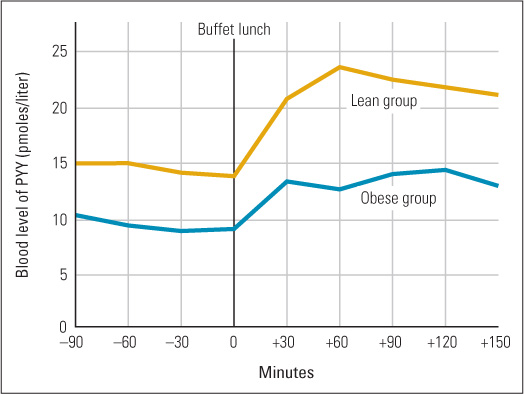
In both rats and humans, injection of extra PYY into the bloodstream reduces total food consumed over the next several hours (Gardiner et al., 2008). In one double-blind experiment with humans, PYY injection reduced the amount of food eaten at a buffet luncheon, by both lean and obese human volunteers, by an average of about 30 percent, and also reduced reported level of appetite in both groups (Batterham et al., 2003). The same researchers also found that lean subjects had higher baseline levels of naturally produced PYY than did obese subjects and exhibited a much greater increase in PYY following a meal (see Figure 6.5). This result suggests that insufficient PYY production may be a contributing cause of obesity. As you can well imagine, pharmaceutical companies are currently investigating the possibility that PYY, or some modified form of it, might be developed and marketed as a weight-control drug (Murphy & Bloom, 2006).
Leptin Contributes to the Long-Term Control of Appetite and Body Weight
14
How does the hormone leptin contribute to weight regulation, and why isn’t leptin a good anti-obesity drug?
In the short term, eating provides an immediate supply of building blocks (such as amino acids) and energy molecules (such as glucose) needed to grow, repair, and fuel the body. Over the long term, eating more than is immediately needed adds to the amount of fat that is stored in special fat cells in various tissues of the body. Fat stores provide an extra source of energy that the body can call upon when food is not available in the environment. Too much fat, however, impedes movement and puts stress on all the body’s organs. Not surprising, the hunger mechanism, when it is working optimally, is sensitive not just to the short-term indicators of the amount of food recently eaten but also to the amount of fat stored in the body.
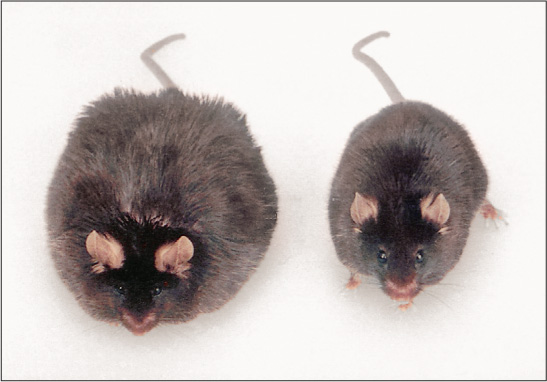
Fat cells in mice, humans, and other mammals secrete a hormone, called leptin, at a rate that is directly proportional to the amount of fat that is in the cells (Woods et al., 2000). Leptin is taken up into the brain and acts on neurons in the arcuate nucleus and other parts of the hypothalamus to reduce appetite. Animals that lack either the gene needed to produce leptin or the gene needed to produce the receptor sites for leptin in the hypothalamus become extraordinarily obese (see Figure 6.6) (Friedman, 1997). Some people—though very few—lack the gene needed to produce leptin. Such individuals are extremely obese, but they reduce their eating, lose weight rapidly, and keep it off when given daily injections of leptin (Farooqi et al., 2002).
208
When the hunger-suppressing effect of leptin was discovered in the 1990s, there was much excitement about the possibility that injections of this hormone could help many people lose weight. Subsequent research, however, proved otherwise. The great majority of overweight people are not lacking in leptin. Their fat cells constantly produce very high levels of the hormone. Research shows that hunger is reduced by increased leptin up to a certain level, but most overweight people already have blood concentrations of leptin well above that level, and additional leptin has no effect (Marx, 2003). Other research suggests that many obese people feel chronically hungry not because they lack leptin but because their brains are relatively insensitive to the hormone (Berthoud & Morrison, 2008; Friedman, 2003). A drug that helped restore leptin sensitivity might help them lose weight, and such a drug has been found effective in suppressing appetite in mice (Tam et al., 2012), but so far the drug has not been tested with humans.
Roles of Sensory Stimuli in Control of Appetite
15
How do conditioned stimuli and the availability of many foods, with different flavors, contribute to appetite and obesity?
As you probably know from personal experience, hunger is provoked not just by events inside us but also by sensory stimuli in the environment. Even if you were not initially hungry, the sight or smell of good food might make you hungry. This effect of the environment makes good sense from an evolutionary perspective. For most of our history as a species, food was not always available. Evolution led us and other animals to be opportunists with regard to food; our hunger increases when food is available so that we don’t pass up opportunities to eat when eating is possible.
Through classical conditioning (discussed in Chapter 4), any cues that have previously signaled opportunity to eat—such as the sight or smell of good food, the sound of a dinner bell, or the sight of a clock showing it is dinner time—can bring on a sudden surge of appetite. Such conditioning is reflected not just in reports of increased hunger but also in the occurrence of reflexive physiological responses, such as the secretion of saliva and digestive juices, that help to prepare the body for food and add further to the sense of hunger (Woods et al., 2000).
Once a person begins to eat, the taste of the food can influence the reduction or prolongation of appetite during a meal. People and laboratory animals that eat a type of food until they are satiated experience renewed appetite when a different food, with a different taste, is placed before them. This phenomenon is referred to as sensory-specific satiety, and many experiments show that it is mediated primarily by the sense of taste (Raynor & Epstein, 2001). When people eat one food at a meal, their rating of the taste pleasantness of that food declines relative to their rating of the taste pleasantness of other foods. This effect begins immediately after eating the food and lasts typically for several hours. Experiments with animals show that the sight and smell of a new food can result in renewed activity in appetite-stimulating neurons in the hypothalamus after the animal has been sated on a different food (Rolls et al., 1986). Laboratory animals that can regularly choose from a variety of different-tasting foods eat more, and become fatter, than do animals that have only one food choice, even if the nutritional content of various foods is identical (Raynor & Epstein, 2001). People, too, eat more when there are more food choices (Raynor & Epstein, 2001; Temple et al., 2008).
209
Problems of Obesity
Human evolution occurred almost entirely in environments where food choices were far fewer, and far lower in fat and sugar, than are the foods available to people in modern cultures. Natural selection built into us excellent mechanisms to defend against weight loss in times of scarcity, but rather poor mechanisms to defend against obesity in times of plenty. Obesity is a cultural disease of our time and place.

To assess obesity, the World Health Organization uses a measure called the body mass index, or BMI, defined as body weight in kilograms divided by the square of the person’s height in meters. A BMI of 25 or more is considered overweight, and one of 30 or more is considered obese. Thus a person who is 1.7 meters (5 feet 7 inches) tall and weighs 73 kilograms (161 pounds) is deemed overweight (BMI = 73/1.72 = 25.3), and a person of that same height who weighs 88 kilograms (194 pounds) is considered obese (BMI = 88/1.72 = 30.4).
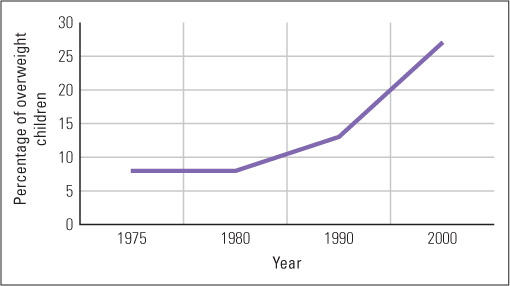
The BMI does not take into account one’s gender, age, or how muscular or flabby a person’s body is, thus it provides only a general measure to evaluated obesity. Nevertheless, using the BMI, a survey taken in 2009–2010 reported that 69 percent of adults in the United States were overweight, and more than half of those who were overweight were obese (Flegal et al., 2012). The overweight epidemic is not limited to adults, but is found increasingly in children. For data collected in 2009–2010, 32 percent of all U.S. children between 2 and 19 years old were overweight, with 17 percent being obese (Ogden et al., 2012). Comparison of these findings with those of studies done earlier (for example, Hill et al., 2003) reveal that the rate of obesity has risen rapidly over the past few decades and is also rising rapidly in many other parts of the world. (See Figure 6.7 for changes in childhood obesity in England between 1975 and 2000.) As obesity rises, so does the rate of diseases that are secondary to it, including Type 2 diabetes, coronary heart disease, stroke, and certain types of cancers (Marx, 2003). In the United States, obesity is rapidly overtaking smoking as the major underlying cause of death (Marx, 2003). The immediate causes of this obesity epidemic are clear: People consume more calories and exercise less than they used to.
Effects of Genes and Nutrition on Body Weight
16
What is the evidence that within a culture, differences in body weight result mostly from differences in genes, but across cultures, environment plays a large role?
Within the United States, or any other Western culture, the determination of who does or does not become obese depends very much on genes and relatively little on the specific home environment (Barsh et al., 2000). The weights of adopted children correlate much more strongly with the weights of their biological parents than with those of their adoptive parents; identical twins usually have very similar weights, even if raised in different homes; and pairs of biological siblings raised in different homes are, on average, nearly as similar to each other in weight as are pairs raised in the same home (Grilo & Pogue-Geile, 1991; Stunkard et al., 1986). This does not mean that body weight is little influenced by the environment. It simply means that the environmental conditions that promote obesity are fairly constant within Western cultures, so differences in weight have mostly to do with genetic differences in how individuals respond to those conditions.
210

Across cultures, environmental differences can have a large effect on body weight. As one example, obesity is very common among Pima Indians living in Arizona but is essentially absent among their genetic relatives living in Mexico. The Mexican Pimas subsist mainly on grains and vegetables; their culture does not include the high-calorie foods available to the American Pimas (Gibbs, 1996). The genes that promote obesity in our culture apparently do so by increasing the person’s attraction to high-calorie foods, by decreasing one or another of the feedback effects that high food intake or fat level has on the hunger mechanisms in the hypothalamus, and by decreasing the body’s ability to burn up excess calories quickly (Barsh et al., 2000; Friedman, 2003). Where high-calorie foods are unavailable, the same genes generally don’t lead to obesity.
The diets of people in the developed world are different from diets of traditional people in many ways; however, the prevalence of sugars in modern diets, particularly fructose, has been a topic of considerable debate. Fructose, which is found in sucrose and high-fructose corn syrup, has been identified by some to be a particularly potent source of calories and a major contributor to the obesity problem. For example, one recent study investigated the brain’s reactions to fructose versus glucose, as well as subjects’ perception of satiety after consuming these sugars (Page et al., 2013). The researchers asked 20 healthy, normal-weight young adults to consume 75 grams of either pure fructose or pure glucose and then measured their brains’ reactions via fMRI. They reported differences in activation of various areas of the hypothalamus between the fructose and glucose conditions, as well as differences in the striatum, a subcortical area involved in inhibitory responses. More important, subjects reported feelings of fullness and satiety after consuming glucose, but not after consuming fructose. Although this is a preliminary study with a small sample that used levels of sugar ingestion far in excess to what an individual would normally consume at a single time, it illustrates that, at least under some conditions, all sugars are not alike as far as the brain is concerned, and that fructose, found in abundance in many prepared foods, may be a particular problem when it comes to obesity.
Although diets rich in sugars and fats are the main culprit of obesity in modern cultures, modern life is also marked by a decrease in physical activities. Fewer adults work at jobs that require physical exertion, and more people engage in sedentary recreational activities, such as watching television and playing video games, than did our ancestors. This tendency toward a less active lifestyle begins in childhood. Schools have been cutting back on both recess and physical education since the 1960s (Nestle & Jacobson, 2000; Pellegrini, 2005), and outdoor play in general has decreased.
Another factor involved in obesity is prenatal nutrition. Women with poor diets when pregnant are more apt to have children who are overweight. Such infants typically have lower birth weight than infants with better prenatal nutrition. While most eventually catch up to their peers in weight, they show elevated levels of the appetite-regulating hormone leptin. They develop “thrifty phenotypes,” storing more fat than children whose prenatal diets were more nutritious. This makes sense from an evolutionary perspective, with fetuses being sensitive to their level of nutrition. When prenatal nutrition is good, brain circuitry that controls appetite and metabolism develops as if food resources will be plentiful in the future; when prenatal nutrition is poor, brain circuitry develops differently, causing individuals to hold on to as many calories as they can in anticipation of limited food resources (see Gluckman & Hansen, 2005). Peter Gluckman and Mark Hanson (2005) refer to fetuses responding to current conditions (in this case poor nutrition) not for immediate advantage but in anticipation of later advantage after birth as predictive adaptive responses. The result of this strategy with respect to poor fetal nutrition for people in modern cultures is often obesity.
211
Problems of Dieting
It might seem that the problem of obesity could be solved by a simple application of will power on the part of individuals who weigh more than is healthy, but this is far easier said than done. Weight gained is often very difficult to lose. Decreased food intake not only activates the hunger mechanisms in the brain but can also produce a decline in basal metabolism (the rate at which calories are burned while the individual is at rest), making the body convert food more efficiently to fat (Keesey & Corbett, 1984; Leibel et al., 1995). In one extreme case, a woman managed to reduce her weight from 312 pounds to a still-obese but much healthier 192 pounds through diet. She maintained her new weight for at least 18 months without losing any more by eating a total of 1,000 to 1,200 calories a day—less than half of what most women would have to eat to maintain that weight (Rodin et al., 1989).
17
On the basis of the reports of successful dieters and the advice of appetite researchers, what can people do to maintain a lower weight?
Despite the odds, some people do lose significant amounts of weight and avoid regaining it. A study of approximately 3,000 highly successful dieters, who had lost an average of over 60 pounds per person and had kept it off for an average of 5 years at the time of the study, revealed that they succeeded primarily by avoiding high-fat foods and by greatly increasing their exercise (Butler, 2004; Wing & Hill, 2004). Many other studies, too, have shown that a combination of exercise and dieting is far more effective in producing long-term weight loss than is dieting alone (Cudjoe et al., 2007). Regular exercise not only burns up calories immediately but also builds muscle, which, even when resting, burns calories at a higher rate than do other body tissues (Van Itallie & Kissileff, 1990).
Researchers who study appetite and metabolism often have some sensible advice for people who want to maintain a healthy weight. Here is a summary of what we have gleaned from their work:
- Don’t try to lose weight rapidly. Don’t go on a diet that you can’t stay on for the rest of your life. Weight rapidly lost through starvation dieting is rapidly regained when the diet ends.
- Instead of reducing food intake to a level that leaves you hungry, shift the foods you eat away from high-calorie fats and sweets and toward gut-filling but low-calorie vegetables, fruits, and complex carbohydrates (whole-grain breads and cereals).
- Make the sensory-specific satiety effect work for you instead of against you. Provide yourself, over time, with a luscious variety of vegetables and fruits and very few choices of high-calorie meats and dairy products; and omit pastries, soda pop, potato chips, and French fries entirely.
- Eat each meal slowly, so as to enjoy it and to provide time for the food’s satiety-producing effects to develop. If you still feel hungry after eating what you initially planned to eat, wait at least 15 minutes before deciding whether or not to eat more. By that time you may no longer feel hungry. It takes about 15 minutes for PYY and other satiety-producing consequences of a meal to begin to exert their effects on the brain’s arcuate nucleus.
- If you have a sedentary job or are a student, develop some pleasurable hobbies that involve muscle-building exercise for at least a few hours a week. (Neither of us can stand jogging and get bored with weight lifting, but we love bicycling, kayaking, and cross-country skiing [Peter Gray] and basketball [David Bjorklund].) And when you need to get from one place to another, use your muscles rather than an automobile or an elevator to convey you whenever you can.
Through such changes in habits, many people can lose a significant amount of weight and keep it off for a lifetime, without feeling deprived at all.
SECTION REVIEW
Hunger is a regulatory drive controlled by neural, hormonal, and sensory factors.
Control Mechanisms of Hunger
- The arcuate nucleus of the hypothalamus is a feedback-based appetite control center, with both appetite-stimulating and appetite-suppressing neurons.
- Eating a large meal causes physiological changes, including the release of PYY, that influence the arcuate nucleus and nearby areas to reduce hunger.
- Leptin, a hormone produced by fat cells, helps to regulate body weight by acting on the hypothalamus to reduce appetite.
- Sensory stimuli also affect appetite, as illustrated by sensory-specific satiety and by the appetite-boosting power of learned cues that signal the availability of food.
Obesity
- Within a culture, genetic differences are the primary determinants of who becomes obese, but across cultures, environmental differences play a substantial role.
- Decreasing food intake activates hunger mechanisms in the brain and can reduce basal metabolism, making weight loss harder.
- Poor prenatal nutrition can lead to fetuses developing “thrifty phenotypes,” anticipating future environments of food scarcity by storing more fat than children whose prenatal diets are more nutritious.
- Developing good nutrition habits and engaging in regular exercise are among the techniques that can make it easier to maintain a healthy weight.