6.4 Sex: An Example of a Nonregulatory Drive
18
Why is caution advised in extrapolating from laboratory animals to humans in the study of drives, especially the sex drive?
Just as hunger is the most thoroughly studied regulatory drive, sex is the most thoroughly studied nonregulatory drive. As with hunger, much of the basic research on the physiological basis of the sex drive has been conducted with laboratory animals.
There are limits, of course, in the degree to which we can understand hunger, sex, or any drive in humans by studying it in other animals. Human culture, intellect, sensibility, and capacity for conscious self-control affect our behavior in ways that cannot be studied in laboratory animals. People don’t just eat; they dine, which connotes all sorts of social, cognitive, and aesthetic influences. And people don’t just copulate; they fall in love, compose romantic sonnets, gaze into each other’s eyes over candlelit dinners, swear by the moon to be faithful, have affairs, suffer guilt, and engage in long, intimate discussions with their beloved. Keep in mind as you read that our concern here is with the basic physiological mechanisms that we humans share, more or less, with other mammals, not the whole range of issues concerning human sexuality.

Even when dealing with the copulatory act itself, humans differ quite sharply from rats and other laboratory animals. Among nonhuman mammals, including most other primates, copulation occurs in a stereotyped way, with one set of postures and movements for the female and a different set for the male (see Figure 6.8). Among humans, by contrast, the variety of ways to copulate is limited only by imagination. As you will discover when you read further, humans differ from other species also in the hormonal regulation of the sexual drive, especially in females.
213
In the discussion of hunger, we pointed out that the drive state depends on external conditions—such as the sight and smell of good food—as well as on the internal state of the body. Perceptions and thoughts concerning the external environment are even more obviously crucial to the sexual drive. The attributes and apparent willingness of a potential sexual partner, the perception of safety or danger associated with the behavior, the presence or absence of conditioned stimuli associated with past sexual experiences (discussed in Chapter 4), concerns about the long-term consequences, and so on and so on, all greatly affect the degree to which a person feels sexually motivated at any given moment. In this discussion, though, our focus is on the longer-term effects of hormones on sexual drive. The hormonal regulation of sexual drive differs between males and females, so we’ll discuss such regulation separately for men and women.
Hormonal Influences on Male Sex Drive
Although sexual desire and response are influenced by a number of different hormones, in male mammals, the most crucial hormone for the maintenance of the sexual drive is testosterone, a form of androgen, produced by the testes.
Testosterone Maintains the Capacity for Male Sex Drive
19
What is some evidence that testosterone is needed to maintain the male’s sex drive and that, at least in some species, it does so by direct action in the hypothalamus?
In male animals, castration (removal of the testes, and hence of the main supply of testosterone) causes a marked decline in the sex drive—not all at once, but gradually (Feder, 1984). It takes days to occur in rats, weeks in dogs, sometimes months in monkeys. The injection of testosterone into the bloodstream of castrated animals gradually but eventually fully restores their drive.
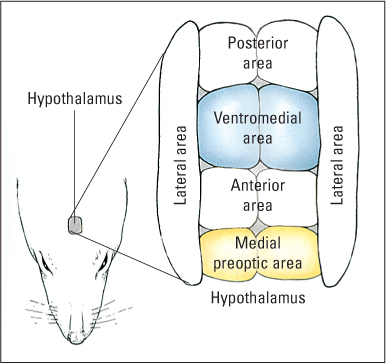
The sex drive can also be restored in castrated male animals by implanting a tiny crystal of testosterone into an area of the hypothalamus called the medial preoptic area (see Figure 6.9) (Davidson, 1980). Neurons in this area contain many receptor sites for testosterone, and small lesions there abolish sexual behavior in male rats (Meisel & Sachs, 1994). Apparently, the medial preoptic area of the hypothalamus is a crucial part of the central drive system for sex in male rats and other male animals that have been studied, and testosterone acts there in a rather prolonged way to enable neural activity to occur and sustain the drive.
Testosterone is also crucial for maintaining the sex drive in human males. Men castrated in an accident or for medical reasons almost always experience a decline (though often not a complete loss) in sex drive and behavior, and testosterone injections restore their drive, usually fully (Money & Ehrhardt, 1972). In other studies, testosterone injections administered to noncastrated men whose testes were producing unusually low amounts of the hormone sharply increased their reported sexual drive and behavior (Davidson et al., 1979; Reyes-Vallejo et al., 2007). At least for many men, the effects of such treatment have more to do with drive than with sexual capability. Low-testosterone men are generally capable of the mechanics of sexual behavior, including erection and ejaculation, but have relatively little desire for it until injected with testosterone (Davidson & Myers, 1988).
214
Causes and Possible Consequences of Increased Testosterone Secretion
20
What kinds of experiences have been shown to increase testosterone production in men? What effects might such increased testosterone have on a man’s subsequent behavior?
Many experiments have shown that the amount of testosterone that men secrete into their blood is affected by psychological conditions. In general, conditions that would seem to promote self-confidence tend to increase a man’s production of testosterone. For example, winning a game, even a sedentary game like chess, commonly results in increased blood levels of testosterone in men, detectable within minutes of the victory (Archer, 2006). Pleasant social encounters with women may also increase testosterone production in men (Roney et al., 2007).
There is some reason to believe that fluctuating levels of testosterone may affect a man’s aggressive and competitive tendencies more than sexual drive per se. On average, men with naturally high testosterone levels are more aggressive and more interested in competition and status than are men with lower levels, and there is some (though mixed) evidence that injections of testosterone can increase indexes of aggressiveness and competitiveness in men (reviewed by Archer, 2006). Moreover, in one experiment, men who showed increased levels of testosterone after losing a game were more likely to want a rematch than were men who showed the more typical decline in testosterone after losing (Mehta & Josephs, 2006). High status and dominance is one route by which men attract women, so an effect of testosterone on competition and status seeking could be an indirect means toward increased sexual behavior.

Hormonal Influences on Female Sex Drive
After puberty, a female’s ovaries begin to secrete the female hormones estrogen and progesterone in a cyclic pattern over time, producing the cycle of physiological changes referred to as the menstrual cycle in humans and the estrous cycle in most other mammals. In both humans and nonhumans, this cycle controls ovulation (the release of one or more eggs so that pregnancy can occur). This cycle of hormones also influences sexual drive.
Effects of the Estrous Cycle in Nonhuman Mammals
21
What evidence indicates that ovarian hormones act directly on the brain to activate the sexual drive in female rats? How do female primates differ from female rats concerning the regulation of sexual drive?
In most mammals, female sexual drive and behavior are tightly controlled by the estrous cycle. The female will seek out opportunities for mating, and will copulate, only at that time in the cycle when she is ovulating and hence capable of becoming pregnant. Removal of the ovaries completely abolishes sexual behavior in most nonhuman female mammals, and injection of hormones can fully restore it. For some species an injection of estrogen alone is most effective, and for others (including rats) a sequence of estrogen followed 2 or 3 days later by progesterone is most effective, a sequence that mimics the natural change of hormones during the estrous cycle.
At least in rats, the ventromedial area of the hypothalamus (see Figure 6.9) plays a role in sexual behavior in the female analogous to that of the preoptic area in the male. Insertion of small amounts of estrogen and progesterone directly into this area brings on sexual behavior in rats whose ovaries have been removed, and lesions in this area abolish sexual behavior in otherwise intact females (Blaustein, 2008; Pleim & Barfield, 1988; Schwartz-Giblin et al., 1989). Apparently the cyclic variation in ovarian hormones acts on the ventromedial area to cause the cyclic waxing and waning of sexual drive.
Many species of monkeys and apes, unlike most nonprimate females, can and sometimes do copulate with males at times in their cycle when they are not fertile, though they are much more likely to seek out and initiate sexual contact with a male when they are fertile (Pawlowksi, 1999; Wallen, 2001). In at least some species of primates, including rhesus monkeys, sexual drive during nonfertile times depends not on ovarian hormones but on testosterone and other androgens. The term androgen refers to a category of hormones, including testosterone, which are produced by the testes in male animals and are normally thought of as “male hormones.” These hormones are also produced at lower levels by the adrenal glands, in females as well as in males.
215
Effects of the Menstrual Cycle in Women
22
What is the evidence that women’s sexual drive depends more on androgens than on ovarian hormones? What evidence suggests, nevertheless, that female sexual drive does increase during the time of ovulation?
Human females exhibit still greater liberation of sexual behavior from cyclic hormonal control than do other primates. Women can experience a high or low sex drive at any time in their hormone cycle. Apparently, in women, hormonal activation of the drive has been taken over largely by adrenal androgens. In clinical studies, women whose ovaries have been removed do not generally report a decline in sexual drive, but most women whose adrenals have been removed do report such a decline; and long-term treatment with testosterone reliably increases sexual desire and satisfaction in such women (de Paula et al., 2007; Guay, 2001; Sherwin & Gelfand, 1987).
Although the ovarian cycle does not control women’s sexual drive, there is evidence that the cycle still does influence it to some degree. A number of studies, using various measures, indicate that women are significantly more motivated sexually at the time in their cycle when they are fertile than at other times. The studies have shown that, on average, women during the fertile phase dress more provocatively, speak in more appealing tones of voice, are relatively more drawn to men with highly masculine features, feel themselves to be more sexually attractive and sexually motivated, and initiate sex more frequently than at other times of their menstrual cycle (Gangestad et al., 2004; Pipitone & Gallup, 2008; Schwarz & Hassebrauck, 2008).
A useful distinction here is that between arousability and proceptivity (Diamond, 2006). Arousability refers to the capacity to become sexually aroused in response to appropriate stimuli (the right partner, the right music, the romantic talk, the gentle touches, and so on), and proceptivity refers to the person’s motivation to seek out and initiate sexual activity, even when sexually arousing stimuli are not already present. The data suggest that arousability remains relatively constant for women over the course of the menstrual cycle, but proceptivity increases during the fertile period. The increased proceptivity might result from the rise of estrogen and/or progesterone during the fertile period, but it could also result from the rise of adrenal androgens. Researchers have found that secretion of adrenal androgens, especially testosterone, increases markedly during the fertile stage of the menstrual cycle (Salonia et al., 2008).
Sexual Differentiation and Determinants of Sexual Orientation
Sex hormones influence sexual drive and behavior through two different kinds of effects on the brain: activating and differentiating. Activating effects are the kinds of effects that we have been discussing so far. They occur around the time of puberty and after, when hormones work on already-developed brain structures to prime, or activate, sexual drive. Differentiating effects, in contrast, occur before and (in some species) immediately after birth and cause the brain to develop in a male or female direction. They are responsible for the biological differences between males and females in sexual drive and orientation. We turn now to a discussion of the role of hormones in differentiating males and females sexually and to the general issue of causes of human differences in sexual orientation.
Brain-Differentiating Effects of the Early Presence or Absence of Testosterone
23
What are some effects of the presence or absence of testosterone before birth on development of the genitals, the brain, and behavior? What has been learned from studies of girls and women born with congenital adrenal hyperplasia?
As noted in Chapter 3, the only initial difference between the two sexes, in all mammals, is that females have two X chromosomes and males have a small Y chromosome in place of the second X. A specific gene on the Y chromosome causes the growth of testes (the male gonads) from structures that would otherwise develop into ovaries (the female gonads) (Page et al., 1987). Before birth the testes begin to produce testosterone, which acts on the brain and other bodily structures of the fetus to steer development in the male direction. The rudimentary genitals of the fetus develop into male structures (including the penis and scrotum) if testosterone is present, and they develop into female structures (including the clitoris and vagina) if testosterone is absent. The testes, in turn, produce mullerian inhibiting substance, which inhibits the male fetus’s mullerian ducts from developing into female reproductive tissue (Lee et al., 1993). Early testosterone also promotes the development of brain pathways involved in the male sex drive and inhibits the development of brain pathways involved in the female sex drive (Gorski, 1996; Simerly, 2002).
216
In order to produce these brain-differentiating effects, testosterone must act within a critical period in the animal’s development. In rats, this period runs from a few days before birth to a day or so after birth. In many other species, including humans, the critical period ends before birth. The critical period for testosterone’s effect on the brain is later than that for its effect on the genitals—a fact that can have some interesting consequences. Because of this difference in timing of critical periods, manipulation of hormones at the appropriate time can produce animals that have the genitals of one sex but the brain structures and behavior of the other sex (Feder, 1984; Ward, 1992).
The capacity for testosterone to at least partly masculinize the brain in human females is well demonstrated by cases of girls born with congenital adrenal hyperplasia (CAH), a rare genetic disorder in which the adrenal glands of the developing fetus produce an overabundance of androgens, including testosterone. Girls born with CAH have partially masculinized genitals. In developed countries, hormone treatments are begun at birth or shortly thereafter to terminate the overproduction of androgens, and surgery is used to feminize the genitals. These girls are raised by their parents as normal girls, but many studies have shown that in various ways they exhibit masculine characteristics. As children, they play more actively and aggressively than do most girls, have more masculine mannerisms, and typically prefer to play with boys and with boys’ toys (Berenbaum & Hines, 1992; Pasterski et al., 2007)—even though their parents give them more positive feedback for playing with girls’ toys than parents of unaffected girls (Pasterski et al., 2005). As adults, they are statistically more likely than other women to be homosexual or bisexual or to report that they are uncomfortable with heterosexual behavior (Hines et al., 2004). These effects seem clearly to be caused by the prenatal androgens and not by any difference in how they were treated during their development.
Perhaps you are wondering why a male hormone, not a female hormone, plays the key role in early sexual differentiation in mammals, including humans. The answer is that the female hormones (progesterone and estrogen) are produced by pregnant females at high levels and get into the tissues of all fetuses, of both sexes. If female hormones promoted growth of female structures during fetal development, all mammalian infants would be feminized. In birds and reptiles—which develop in eggs outside the mother’s body—early sexual differentiation is determined by the presence or absence of estrogen, not testosterone (Adkins-Regan, 1981).
Falling in Love
24
What may be the purpose of romantic love?
Sex among humans is often accompanied by romantic love, or “falling in love.” Falling in love is universal (Jankowiak & Fischer, 1992) and associated with strong emotions, including exhilaration, euphoria, a pounding heart, and increased energy. This is sometimes accompanied by a loss of appetite, anxiety, or sleeplessness (Fisher, 2000, 2004). Romantic love is not necessary for sex to occur, of course, but it often increases its likelihood, and it also serves to form a bond between lovers. Anthropologist Helen Fisher (2000) proposed that human pair bonding evolved as females required increasing protection and resources to care for their slow-developing offspring. It also became in men’s best interest to support their mates and their mates’ children if they expected to pass along their genes to any grandchildren. The survival of their dependent offspring is greatly benefited by paternal support (Geary, 2005b). As a result, it became in both men’s and women’s best genetic interest to work together to rear their offspring, and falling in love is one mechanism to get this process started.
217
Fisher (2000, 2004) proposed that falling in love involves three primary emotional systems that evolved to support mating, reproduction, and parenting: lust, attraction, and attachment. Lust—becoming sexually excited by members of the opposite sex (or by the same sex for someone with a homosexual orientation)—is driven in both men and women primarily by androgen hormones (testosterone being a potent example of an androgen hormone). But lust is not love, nor does it promote long-term attachment. For example, when male sparrows were injected with testosterone, thereby increasing lust, they abandoned their mates to pursue other females (Wingfield, 1994). In humans, there is a small but significant relation between a man’s level of testosterone and the likelihood that he is married: Men with high levels of testosterone are less likely to marry—and have less stable marriages when they do—than men with lower levels (Booth & Dabs, 1993).
The second system for Fisher, attraction, is synonymous with romantic love; it is characterized by the strong emotions described earlier such as exhilaration, increased energy, and loss of appetite. Romantic love is associated with an increase in the neurotransmitter dopamine, high levels of which are related to heightened attention, motivation, goal-directed behaviors, euphoria, and increased mental activity (Fisher, 2000). Romantic love is also associated with increased levels of norepinephrine, which itself is associated with more acute memory for new stimuli and events. The love-possessed focus their attention on their loved one and remember often-trivial details related to them. Fisher speculated that the inability of those in love to keep thoughts of their beloved out of their mind is similar to obsessive-compulsive disorder and associated with low levels of the neurotransmitter serotonin. The elevated levels of neurotransmitters associated with being in love typically return to normal between 6 and 18 months after initially falling in love (Fisher, 2000). This is time enough for pregnancy to occur, but if such passionate love wanes, what is to keep a man and a woman together following the birth of a baby? It is the third emotional system, attachment, which facilitates longer-term relationships necessary to rear children.
Attachment in Fisher’s theory refers to male–female bonding. Men and women who are attached express feelings of closeness, security, anxiety when separated from one’s partner for an extended length of time, and mild euphoria when with one’s partner (Liebowitz, 1983). Such attachment feelings are associated with the hormones vasopressin and oxytocin. These same hormones are released during sexual intercourse and are also associated with infant–mother attachment.
People of all ages show basically the same behavioral and physiological reactions when they fall in love (Fisher, 2004). The mechanisms responsible for falling in love in adolescence and young adulthood seem to operate across the entire life span, even though older adults—and especially women past childbearing age—typically would not need to form a bond lasting long enough to enable them to rear a child to some level of independence. While sexual desire (Tomic et al., 2006) and sexual response (Medina, 1996) do decline in older adults, many older adults remain sexually active, and some do fall in love (Fraser et al., 2004).
218
Effects of Genes and Prenatal Environment on Sexual Orientation
Although sexual orientation would seem to be a simple thing, it is not. It has at least three components:
- sexual/romantic attraction, or arousal (to which sex do people feel most attracted),
- sexual behavior (with which sex do people have voluntary sexual acts), and
- sexual identity (how do people describe themselves: heterosexual, gay, lesbian, or bisexual).
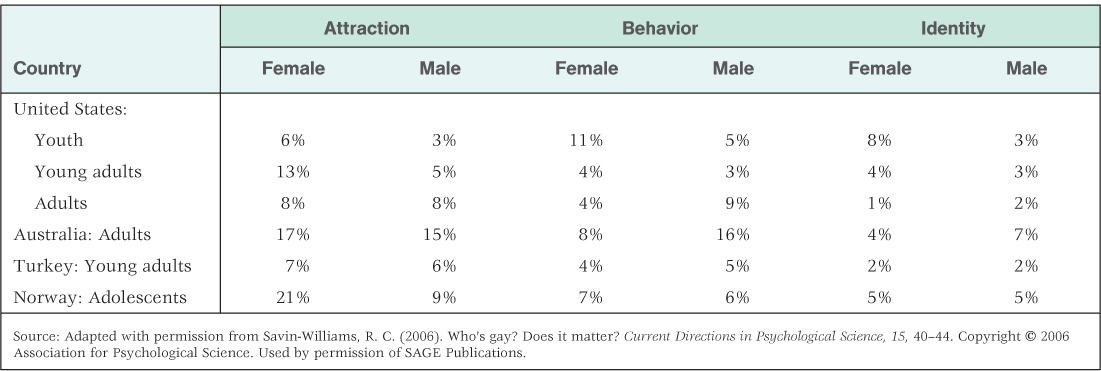
These components are not always highly correlated with one another, so depending on which component one uses, rates of homosexuality can vary greatly. Table 6.1 presents a summary of rates of homosexuality in four countries, based separately on each of the three components of sexual orientation: attraction, behavior, and identity (Savin-Williams, 2006). As you can see, depending on which measure is used, the rates vary between 1 percent and 21 percent. If we describe homosexuality in terms of exclusive attraction to members of the same sex, the estimate is between 3 percent and 7 percent (Epstein, 2006). A possibly larger percentage, even harder to estimate precisely, is bisexuality (attraction to members of both sexes), which appears to be more common in women than in men (Diamond, 2006; Rahman & Wilson, 2003).
Rates of homosexuality in four countries depending on the criteria used: sexual/romantic attraction, sexual behavior, or sexual identity. As you can see, the frequency of homosexuality depends on which criterion you use.
Most people, whether homosexual or heterosexual, experience their sexual orientation as a deep-seated, fundamental part of their being, something they discover about themselves as they are growing up, not something that they have a choice about (Bell et al., 1981; Rahman & Wilson, 2003). In fact, many homosexual men and women show signs of gender nonconformity (that is, engage in opposite-sex behavior) in childhood (Drummond et al., 2008). This was demonstrated in a study that looked at childhood home videos of homosexual and heterosexual men and women. Observers did not know the adult sexual orientation of the children they viewed in the videos. Nevertheless, they rated the pre-homosexual children as engaging in more gender nonconformist behaviors than preheterosexual children. This effect was found for both men and women (Rieger et al., 2008).
219
25
What is the evidence that genes and prenatal environment can influence sexual orientation in humans? What is the effect of fraternal birth order on male sexual orientation, and how might it be explained in terms of the prenatal environment?
Genetic differences among individuals play a significant role in determining sexual orientation, but not the sole role. Several research studies reveal that roughly 50 percent of the genetically identical twin brothers and sisters of homosexual men and women are also homosexual, compared with about 15 percent of the same-sex nonidentical twins or nontwin siblings of homosexual men and women (Hyde, 2005; Rahman & Wilson, 2003). If homosexuality were completely determined by genes, then 100 percent of the identical twin brothers and sisters of homosexuals would be homosexual.

Other studies suggest that sexual orientation might be affected by a variety of prenatal environmental factors—ranging from prenatal stress to certain medications taken by the mother during pregnancy—that alter the amount of testosterone or other androgens available to the fetus’s brain during a critical period in development (Ellis & Cole-Harding, 2001; Ellis & Hellberg, 2005). Such work suggests that a high level of androgen action during the critical period may promote the development of brain mechanisms that predispose sexual attraction toward women and not toward men, and that a relative lack of androgen during the same period may promote the opposite.
The single most consistent, nongenetic influence on sexual orientation discovered to date is the fraternal birth-order effect on male homosexuality (Blanchard, 2008). The more older brothers a man has, the greater is the likelihood of his being homosexual. The effect is quite strong. One large study revealed that, on average, a couple’s first son has a 2.0 percent chance of being homosexual and that this percentage increases to about 2.6 percent for a second son, 3.4 percent for a third son, 4.6 percent for a fourth son, and 6.0 percent for a fifth son (Blanchard, 2001). In contrast, the number of older sisters a man has plays no role in the likelihood of his being homosexual, and there is no birth-order effect on homosexuality in women. Another study revealed that, for boys adopted in infancy, homosexuality increased with the number of older biological brothers, not with the number of older adoptive brothers. In other words, the crucial factor in increasing the likelihood of homosexuality was the number of prior male offspring their biological mothers had produced, not the number of older boys in the family in which they were raised (Bogaert, 2006). This result strongly suggests that the effect derives from the prenatal environment. The most fully developed current hypothesis is that there is a “maternal memory” for male gestations or births. Having a male fetus alters a woman’s immune system. Male fetuses are interpreted as foreign to a woman’s body and as a result she develops some male antibodies, which in turn affects subsequent male fetuses (Blanchard, 2008; Bogaret, 2006).
Possible Effects of Experiences, After Birth, on Sexual Orientation
There was a time, more than three or four decades ago, when most psychologists and psychiatrists believed that homosexuality results primarily from experiences that young people have growing up. Various theories about such environmental causes were proposed, but subsequent interviews and surveys of thousands of homosexuals and heterosexuals failed to support them (Bell et al., 1981; Dawood et al., 2000; Rahman & Wilson, 2003). Such studies revealed little or no evidence that style of parenting, absence of the father or mother, early seduction or rape by someone of the same or opposite sex, or degree of opportunity for one or another type of sexual experience in adolescence contributes significantly to the development of sexual orientation.
26
What evidence suggests that sexual orientation may more often be influenced by postnatal experience and personal choice for women than for men?
However, recent research has revived the idea that experiences in life can affect sexual orientation, especially for women. In general, women appear to be more flexible in their sexual orientation than men. One line of evidence for this comes from direct physiological measures of sexual arousal in response to erotic films (Chivers et al., 2004). Such measures show that most women, whether they are self-declared homosexuals or heterosexuals, can become sexually aroused by erotic films of either men or women engaged in sexual acts. In contrast, men are much more categorical in their arousal: Homosexual men typically respond only to men and heterosexual men typically respond only to women.
220
Lisa Diamond (2006) has argued that because women are capable of sexual arousal to either men or women, they are more capable than are men of switching their sexual orientation at any time in life. Extensive interviews of women, by Diamond and others, seem to bear this out. Women are more likely than men to report that their sexual orientation is a choice, and they are more likely than men to change their sexual orientation in response to events that occur in their lives. For example, some women become homosexual as a result of falling in love with a particular woman. They are not so much attracted to women in general as to one individual woman. Some others (whom Diamond refers to as “political lesbians”) adopt a lesbian identity and behavior because they have become involved with a social group that values lesbianism.
Such interviews also suggest, however, that some homosexual women are less flexible in their sexual orientation than are others. The distinction seems to fall roughly along the line of the popular culture’s distinction between “butch” and “femme” lesbians (Diamond, 2006; James, 2005). The former tend to be more masculine in appearance, more sexually assertive, and more firmly homosexual than the latter. These may generally be women whose brains have been partly masculinized prenatally. The women that Diamond finds are most likely to change their sexual orientation as a result of life experiences are most often those in the “femme” category.
Nothing about human behavior is simple. We are complex creatures. All of our behavior, including our sexual behavior, is determined by many factors, only some of which we have begun to fathom. Beware of any theory of human sexual orientation, or of anything else in psychology, that tries to explain everything in terms of just one simple cause or another.
SECTION REVIEW
Sex is a nonregulatory drive powerfully influenced by hormones.
Male Sex Drive
- Testosterone maintains male sex drive over the long term. In rats, at least, this occurs by action on the preoptic area of the hypothalamus.
- Confidence-boosting events cause increased testosterone secretion in men, which may increase their tendency toward competitiveness or aggression.
Female Sex Drive
- In most nonhuman female mammals, ovarian hormones promote sexual drive at the time of fertility, apparently through action on the ventromedial hypothalamus.
- In women and some other female primates, adrenal androgens promote sexual receptivity throughout the ovarian cycle. But sexual proceptivity appears to increase at the time of fertility, perhaps because of increased androgen production at that time.
Falling in Love
- Falling in love is universal and may have evolved to promote pair-bonding necessary to raise slow-developing offspring.
- Fisher proposed that falling in love involves three primary emotional systems that evolved to support mating, reproduction, and parenting—lust, attraction, and attachment—each associated with patterns of hormone and/or neurotransmitter production.
Sexual Orientation
- In mammals, prenatal androgens masculinize and defeminize the developing brain. This effect may explain some characteristics of girls and women born with CAH.
- Genes influence sexual orientation in both men and women, as does the prenatal environment.
- The fraternal birth order effect on male homosexuality may be mediated by the prenatal environment.
- Women appear to be more flexible than men in sexual orientation.