7.5 Pain
16
In what ways is pain a “body” sense, an emotion, and a drive? How does observation of people born without pain sensitivity illustrate pain’s value?
Pain is one of the somatosenses (introduced in Chapter 5). That is, like touch, temperature sensitivity, and proprioception (the sense of body position), pain is a sense that can originate from multiple places throughout the body rather than just from specialized sensory organs in the head. (Recall that soma means “body.”) Pain receptors exist over the whole surface of the skin and in many other bodily tissues.
Pain is a “body” sense in another way, too. When you see, hear, smell, taste, or touch something, you experience the sensation as coming from outside yourself (from the thing you are seeing, hearing, smelling, tasting, or touching); but when you feel pain, you experience it as coming from your own body. If you cut yourself with a knife, your feeling of pain is a sense not of the knife (which you experience with vision and touch) but of your own injured bodily state.
Pain is not only a sense but also a perception, an emotion, and a drive. As an emotion, strong pain overwhelms a person’s conscious mind, making it hard to think about anything except the pain; and, like many other emotions, pain has its own well-recognized facial expression (Williams, 2002). As a drive, pain motivates a person both to reduce the pain and to avoid future behaviors like the one that produced it (such as careless handling of knives). In psychology, pain is by far the most thoroughly studied of the somatosenses. That, no doubt, is largely due to the dramatic ways that pain can affect, and be affected by, a person’s other psychological experiences.
The evolutionary value of pain—its role in promoting survival—is dramatically illustrated in those rare, unlucky people who are born with a genetic disorder that makes them insensitive to it (Brand & Yancey, 1993; Cox et al., 2006). They can experience all other skin sensations—including touch, warmth, cold, tickle, and pressure on the skin—and they can report increasing intensities of those sensations, but pain, with its warning and motivating qualities, is missing. Children with this disorder are not motivated to remove their hands from hot stoves, or to refrain from chewing on their tongues as they eat, or to change their body positions (as most of us do from minute to minute) to relieve the strain on muscles and joints. Even if they are constantly watched throughout childhood, and even if they learn intellectually to avoid certain activities, people with this disorder usually die young from the tissue deterioration and infections that result from their wounds.
Neural Pathways for Pain
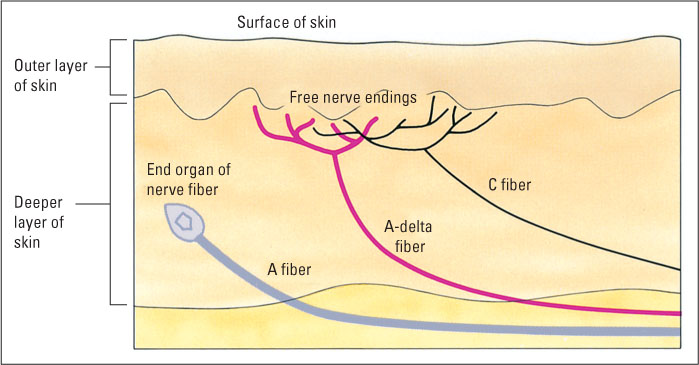
The anatomical basis of pain is closely related to that of the other somatosenses, such as touch and temperature sensitivity (Hendry & Hsiao, 2012b). For all these senses, the receptor cells are the sensory neurons themselves. These neurons have receptive endings in the skin and long axons that enter the central nervous system. Pain neurons are thinner than other neurons from the skin, and their sensitive terminals, called free nerve endings, are not encased in special capsules, or end organs, as are the endings of touch and temperature receptors (see Figure 7.8). Free nerve endings can be found in all body tissues from which pain is sensed (Lynn & Perl, 1996)—not just the skin but also the pulp of the teeth (from which comes the dreaded toothache), muscles (giving us the pain of cramps and muscle aches), membranes around bones and joints (the pain associated with arthritis), and various visceral organs (giving us stomachaches and other inner pains).
265
Sensory Neurons for Two Waves of Pain
17
What is the anatomical basis for a distinction between first and second pain?
Pain sensory neurons are of two general types—very thin, unmyelinated, slow-conducting neurons called C fibers and slightly thicker, myelinated, faster-conducting neurons called A-delta fibers (see Figure 7.8). Some A-delta fibers are specialized to respond to strong pressure (such as from a pinprick), while others are specialized to respond to extremes of temperature (hot or cold). C fibers respond to all sorts of stimuli that produce pain, including strong pressure, intense heat or cold, and chemicals that produce pain when applied to the skin (Basbaum & Jessell, 2000). When your skin is pricked or burned, you feel two separate waves of pain: a sharp, highly localized first pain, followed (1 or 2 seconds later) by a dull, burning, more diffuse, longer-lasting second pain. The first is mediated by A-delta fibers, and the second by the slower C fibers (Basbaum & Jessell, 2000). The C fibers also respond in a more prolonged way to a variety of chemicals that are released by damaged or infected cells, accounting for the persistent pain that accompanies burns, wounds, and infections.
Pain neurons enter the spinal cord (via a spinal nerve) or the brainstem (via a cranial nerve) and terminate there on interneurons. Some of these interneurons promote reflexive responses—such as the automatic withdrawal of the hand from a hot stove—independent of conscious experience. Others send their axons to the thalamus, in the center of the brain, which, in turn, sends output to portions of the brain that are involved in the conscious experience of pain.
Brain Areas for Three Components of Pain Experience
18
What are three different components of pain experience, and what evidence links these to three different portions of the brain?
Pain as a psychological experience can be divided meaningfully into three different components, each of which depends most critically on a different portion of the brain (see Figure 7.9):
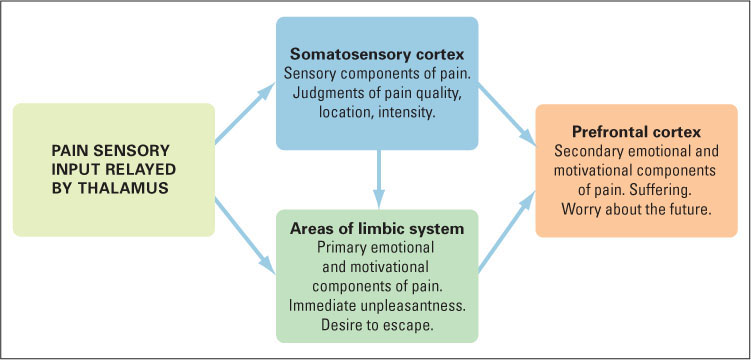
- The sensory component of pain depends largely on the somatosensory cortex, the area of the parietal lobe that receives input for touch and temperature as well as pain (for its location, refer back to Figure 7.1, on page 247). This area appears to be crucial for the ability to perceive pain as a sensation, to describe its intensity and qualities (as sharp or dull, for example), and to locate it in a particular portion of the body.
- The immediately experienced primary emotional and motivational component of pain depends on portions of the limbic system referred to as the cingulate cortex and the insular cortex, which lie buried in the folds of the brain’s frontal lobe. People with damage to these brain regions experience a condition called asymbolia for pain (Price, 2000). They can perceive a painful stimulus and describe it as such, identify the location of the pain, describe its qualities, and rate its intensity; but they do not feel a normal desire to escape the pain. The pain doesn’t bother them.
- A third component of pain, in humans, is the more cognitively based secondary emotional and motivational component—the suffering that derives from the person’s worrying about the future or about the meaning of the pain. The brain area that is crucial for this troubled state lies in the prefrontal lobe, the front-most portion of the cerebral cortex, which is involved in all aspects of planning and concern for the future (as noted in Chapter 5). People with damage here feel and respond to the immediate threat and unpleasantness of pain, but they do not worry about it, just as they do not worry about or make plans based on other experiences (Price, 2000).
266
The experience of pain, with all three of the above components, does not always originate from stimulation of pain receptors. This fact is all too well known by many people who have had a limb amputated. Such people often feel as if the missing limb were still present and full of pain. Such phantom-limb pain can persist even if all the nerves from the limb’s stump have been destroyed and even if the pain pathways entering the brain from the spinal cord have been destroyed (Flor et al., 2006; Melzack, 1992). Apparently, in such cases the brain’s mechanism for experiencing pain and assigning that experience to a particular body location can be activated without sensory input from that part of the body. In fact, the lack of sensory input might trigger phantom-limb pain by removing a source of inhibition to the pain mechanisms of the brain.
The Modulation of Pain
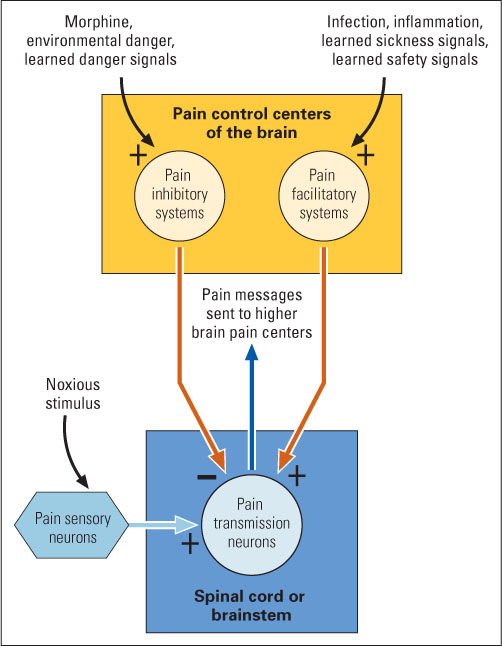
The experience of pain depends not just on the physical stimulus or its damaging effects but also on other conditions that exist at the time the stimulus or damage occurs. The same degree of wound may at one time feel excruciatingly painful and at another time be barely detected. The gate-control theory of pain (Melzack & Wall, 1965, 1996) aims at explaining such variability. In essence, the theory holds that the experience of pain depends on the degree to which input from pain sensory neurons can pass through a neural “gate” and reach higher pain centers in the brain. Conditions can increase or decrease pain by opening or closing the gate. Much research has confirmed this general theory and has added many details to it.
The major gate, where pain input is most strongly enhanced or inhibited, is at the first way station in the pain pathway in the central nervous system. Pain sensory neurons enter either the spinal cord (by way of a spinal nerve) or the brainstem (by way of a cranial nerve) and terminate there on second-order pain neurons that send signals upward, to higher brain areas that enable the experience of pain. The responsiveness of these second-order neurons to pain input is controlled, in part, by pain-enhancing and pain-inhibiting neurons that extend their axons down from higher portions of the brain. The effects of these descending neurons, in the spinal cord and brainstem, are metaphorically referred to as opening and closing the “gate” for pain. Pain-enhancing neurons tend to open the gate, and pain-inhibiting neurons tend to close it, as illustrated in Figure 7.10. The intensity of pain sensation experienced depends on the stimulus and on the balance of forces tending to open or close the gate (discussed in the following paragraphs).
267
Mechanisms of Pain Enhancement
19
How does illness produce a general increase in pain sensitivity, and how does injury produce a localized increase in pain sensitivity?
Recall the last time you were ill. Chances are that you felt increased pain sensitivity all over your body, especially if you had a high fever. Evolutionary psychologists hypothesize that this pain enhancement effect may have evolved to motivate ill individuals to rest rather than move around, in order to conserve energy needed to fight the disease (Kluger, 1991). Although the details are not fully understood, this illness-induced effect is believed to occur through an action of the immune system on pain-enhancing neurons in the brain (again, see Figure 7.10) (Watkins & Maier, 2000; Watkins et al., 2007).
Pain sensitivity can also be increased in specific locations of the body as a result of injury at those locations. This heightened sensitivity occurs partly because of changes in the free nerve endings of C fibers and A-delta fibers that are induced by chemicals released from damaged cells (Basbaum & Jessell, 2000). The sensitized sensory neurons respond to much weaker stimuli than they would have before the injury. In addition, second-order pain neurons in the spinal cord and brainstem become sensitized by intense activation, such that they become more responsive to subsequent input for periods ranging from minutes to weeks (Ji et al., 2003; Woolf & Salter, 2000). The result is that even light touch to a recently burned or wounded area of skin can be intensely painful. Such pain-enhancing systems presumably evolved as a means of motivating individuals to protect damaged areas of their bodies.
Neural and Chemical Mechanisms of Pain Reduction
20
How can pain input be inhibited at its entry into the central nervous system, and how might endorphins be involved in this process?
In addition to understanding the mechanisms that exacerbate pain and pain sensitivity, it is also important to understand how pain can be reduced, or inhibited. A major neural center for pain inhibition exists in a portion of the midbrain called the periaqueductal gray (abbreviated PAG). Neurons in this area send their axons down into the lower brainstem and spinal cord to inhibit pain input there, as illustrated in Figure 7.10. Electrical stimulation of the PAG has a powerful analgesic (pain-reducing) effect—so powerful, in fact, that abdominal surgery can be performed without drugs in animals that are receiving such stimulation (Mason, 2001; Reynolds, 1969). In humans, electrical stimulation of this area has also successfully reduced or abolished chronic pain that could not be relieved by other means (Hosobuchi et al., 1979; Perlmutter & Mink, 2006).
Morphine and other opiate drugs (derivatives of opium) exert their well-known analgesic effects partly through direct action in the PAG. Morphine that passes into the brain is taken up at special binding sites on neurons in the PAG, where it increases neural activity and thereby reduces pain (Basbaum & Fields, 1984). Morphine binding sites are also found on neurons in the spinal cord, and injection of a tiny amount of morphine directly into the spinal cord can greatly reduce or eliminate pain in the part of the body that sends its sensory neurons into that spinal cord area (Basbaum & Jessell, 2000).
Of course, the pain-inhibiting system did not evolve to respond specifically to morphine or other foreign substances. Its basic function is to mediate the body’s own capacity to reduce pain. The body produces a variety of chemicals that act like morphine and are collectively referred to as endorphins. (Recall from Chapter 6 that endorphin is short for endogenous morphine-like substance) Some endorphins are produced in the brain or spinal cord and serve as neurotransmitters to alter the activity or excitability of neurons to which they bind. Others are secreted from the pituitary and adrenal glands as hormones, which enter the bloodstream and have a variety of effects both peripherally and in the central nervous system (Henry, 1986). Endorphins are believed to inhibit pain by acting both in the PAG and at the places where pain-carrying neurons enter the spinal cord and lower brainstem.
Stress-Induced Analgesia
During his search for the source of the Nile River, the famous explorer David Livingstone was attacked by a lion. He survived the incident and later wrote that although the lion shook him “as a terrier does a rat” and crushed his shoulder, he had felt “no sense of pain nor feeling of terror, though quite conscious of all that was happening” (Livingstone, 1857). Other people have had similar experiences. For example, soldiers severely wounded in battle often do not notice their wounds until the battle is over. We are apparently endowed with a mechanism that prevents us from feeling pain at times when, for survival purposes, it is best to ignore our wounds. Faced with a predator or similar threat, a human or other animal cannot afford to nurse a wound or favor it by limping; all the body’s resources must be used to fight or flee. The decreased pain sensitivity that accompanies highly stressful situations is known as stress-induced analgesia.
268
21
What is some evidence that stress-induced analgesia is at least partly mediated by endorphins?
Many experiments have shown that stress-induced analgesia depends partly, if not entirely, on the release of endorphins. In one experiment, rats that were subjected to a series of electric shocks to their feet (the source of stress) became relatively insensitive to pain for several minutes afterward, as indicated by their lack of response to normally painful heat applied to their tails (Lewis et al., 1980). Rats treated with a drug that blocks the action of endorphins did not show this stress-induced analgesia, indicating that the effect must have been mediated by endorphins. In similar experiments, the mere presence of a cat (Lichtman & Fanselow, 1990) produced analgesia in rats, and the presence of biting flies produced analgesia in mice (Kavaliers et al., 1999). In humans, a stressful math test produced analgesia in students (Bandura et al., 1988), and films depicting combat-produced analgesia in veterans who had experienced the trauma of war (Pitman et al., 1990). In all of these cases injection of a drug that blocks the actions of endorphins prevented the analgesic effect from occurring.
Endorphins are also secreted during periods of prolonged, strenuous physical exertion, such as long-distance running, and may account for the pain reduction and euphoric “runner’s high” that many people enjoy during and after such exertion. In one experiment both the reduced pain and the sense of euphoria failed to occur in runners who had been treated with an endorphin-blocking drug (Janal et al., 1984).
Belief-Induced Analgesia
22
What is some evidence that pain can be reduced by belief?
In humans, dramatic reduction in pain can also be produced by the power of belief or faith. Some religious groups engage in practices that most of us would regard as torture, yet the participants appear to feel no pain. One group in India, for example, practices a hook-hanging ritual. A man who has been chosen to represent the power of the gods is secured to a rope by two steel hooks that pierce the skin and muscles on his back. He hangs from this rope, swinging back and forth, while he blesses the children and the crops of the village. He feels honored to have been chosen and apparently feels little or no pain (Melzack & Wall, 1996).
A less dramatic example, in cultures where faith is more often placed in science and medicine, is the placebo effect on pain. In many cases a pill or injection that contains no active substance (the placebo) can reduce pain in a person who believes that the drug is a painkiller. A number of experiments have shown that placebo-induced pain reduction depends partly, and in some cases entirely, on the secretion of endorphins (Price et al., 2008). In one of the first such experiments, people who had undergone a tooth extraction reported less pain if given a placebo than if not, and this placebo effect did not occur in subjects who were treated with an endorphin-blocking drug (Levine et al., 1979). Other experiments have shown that various cognitive techniques for relieving pain, such as meditating on the idea that the pain is disconnected from the rest of the body, also work at least partly through endorphins (Bandura et al., 1987). Might the man hanging from hooks in India also be secreting large quantities of endorphins? Nobody knows for sure, but most pain researchers would bet that he is.
269
SECTION REVIEW
Pain is an emotion and drive as well as a somatosense.
Basic Facts of Pain
- Pain receptors are free nerve endings of pain sensory neurons, Located in many parts of the body.
- C fibers and A-delta fibers, two types of pain sensory neurons, mediate two different waves of pain.
- The experience of pain has three identifiable components—sensory, primary emotional and motivational, and secondary emotional and motivational—each relying on different areas of the brain.
Pain Modulation
- The gate-control theory maintains that the degree of pain felt depends on how much input from pain neurons passes through a neural “gate” to higher areas of the brain.
- The enhanced pain sensitivity that accompanies illness or injury helps protect the body from further harm.
- The PAG and endorphins provide the body with a means of pain inhibition.
- Endorphins play a part in stress-induced and belief-induced analgesia.