Le Châtelier’s Principle

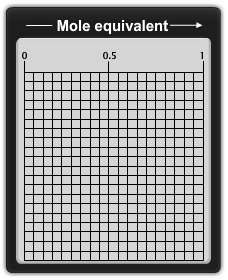





This animation demonstrates Le Châtelier’s principle as it is applied in an esterification reaction.

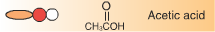


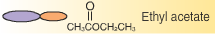

9.03

9.03

9.03

9.03


























Le Châtelier’s Principle
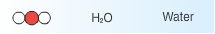
 Le Châtelier’s principle states that a system at equilibrium, which undergoes a change in pressure, temperature, or concentration, will shift to counteract that change until equilibrium is re-established. In an esterification reaction the concentration of ethanol can be changed to affect the yield of ethyl acetate produced in the reaction. |
 0% |
Le Châtelier’s Principle |