Concept 2.1: Atomic Structure Is the Basis for Life’s Chemistry
Living and nonliving matter is composed of atoms. Each atom consists of a dense, positively charged nucleus, with one or more negatively charged electrons moving around it. The nucleus contains one or more positively charged protons, and may contain one or more neutrons with no electrical charge:
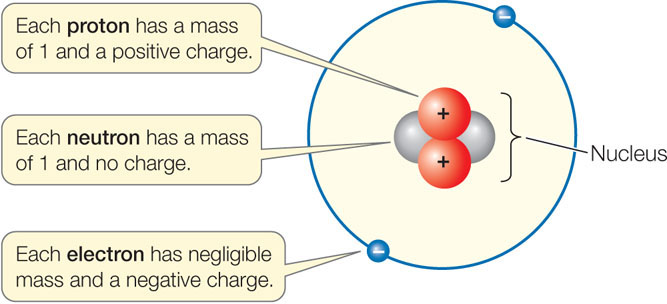
Charges that are different (+/−) attract each other, whereas charges that are alike (+/+, −/−) repel one another. Most atoms are electrically neutral because the number of electrons in an atom equals the number of protons.
The standard unit of measure for the mass of an atom (atomic mass) is the dalton—named after the English chemist John Dalton. A single proton or neutron has a mass of about 1 dalton (Da), which is 1.7 × 10−24 grams, but an electron is even tinier, at 0.0005 Da (9 × 10−28 g). Because the mass of an electron is only about 1/2,000th of the mass of a proton or neutron, the contribution of electrons to the mass of an atom can usually be ignored when chemical measurements and calculations are made.
An element consists of only one kind of atom
An element is a pure substance that contains only one kind of atom. The element hydrogen consists only of hydrogen atoms, the element gold only of gold atoms. The atoms of each element have characteristics and properties that distinguish them from the atoms of other elements.
There are 94 elements in nature, and at least another 24 have been made in physics laboratories. Most of the 94 natural elements have been detected in living organisms, but just a few predominate. About 98 percent of the mass of every living organism (bacterium, turnip, or human) is composed of just six elements:
Carbon (symbol C)
Oxygen (O)
Hydrogen (H)
Phosphorus (P)
Nitrogen (N)
Sulfur (S)
The chemistry of these six elements will be our primary concern in this chapter, but other elements found in living organisms are important as well. Sodium and potassium, for example, are essential for nerve function; calcium can act as a biological signal; iodine is a component of a human hormone; and magnesium is bound to chlorophyll in green plants.
The physical and chemical (reactive) properties of atoms depend on the numbers of protons, neutrons, and electrons they contain. The atoms of an element differ from those of other elements by the number of protons in their nuclei. The number of protons is called the atomic number, and it is unique to and characteristic of each element. A carbon atom has six protons and thus an atomic number of 6; the atomic number of oxygen is 8. For electrical neutrality, each atom has the same number of electrons as protons, so a carbon atom has six electrons and an oxygen atom has eight.
Along with a definitive number of protons, every element except hydrogen has one or more neutrons. The mass number of an atom is the total number of protons and neutrons in its nucleus. The number of neutrons may vary among atoms of a particular element. For example, carbon atoms with six, seven, and eight neutrons are found in nature. These variants are referred to as isotopes. The most common carbon isotope has six neutrons and a mass number of 12, and is referred to as carbon-12 (often written 12C). The most common oxygen isotope (16O) has eight protons and eight neutrons, and a mass number of 16.

Go to MEDIA CLIP 2.1 The Elements Song
PoL2e.com/mc2.1
Electrons determine how an atom will react
The Bohr model for atomic structure (see diagram in previous section) provides a concept of an atom that is largely empty space, with a central nucleus surrounded by electrons in orbits, or electron shells, at various distances from the nucleus. This model is much like our solar system, with planets orbiting around the sun. Although highly oversimplified (you will learn about the reality of atomic structure in physical chemistry courses), the Bohr model is useful for describing how atoms behave. Specifically, the behaviors of electrons determine whether a chemical bond will form and what shape the bond will have. These are two key properties for determining biological changes and structure.
In the Bohr model, each electron shell is a certain distance from the nucleus. Since electrons are negatively charged and protons are positive, an electron needs energy to escape from the attraction of the nucleus. The farther away an electron shell is from the nucleus, the more energy the electron must have. We will return to this topic when we discuss biological energetics in Chapter 6.
The electron shells, in order of their distance from the nucleus, can be filled with electrons as follows:
- First shell: up to 2 electrons
- Second shell: up to 8 electrons
- Third shell: up to 18 electrons
- Fourth and subsequent shells: up to 32 electrons
20
Electrons fill shells closest to the nucleus before occupying shells farther from the nucleus. FIGURE 2.1 illustrates the electron shell configurations for the six major elements found in living systems.
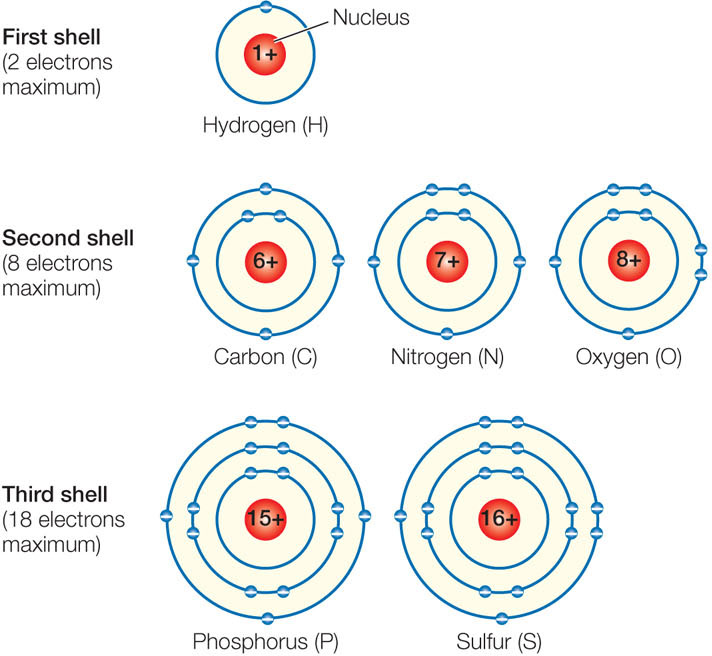
For elements with atomic numbers between 6 and 20 there is a chemical rule of thumb called the octet rule, which states that an atom will lose, gain, or share electrons in order to achieve a stable configuration of eight electrons in its outermost shell. Oxygen, for example, which has six electrons in its outermost shell, will undergo chemical reactions to gain two electrons. When atoms share electrons, they form stable associations called molecules. Most atoms in biologically important molecules—for example, carbon and nitrogen—follow the octet rule. However, very small atoms such as hydrogen (with one proton and one electron) tend to gain, lose, or share electrons such that their single shell contains its maximum number of two electrons.
CHECKpoint CONCEPT 2.1
- What is the arrangement of protons, neutrons, and electrons in an atom?
- Sketch the electron shell configuration of a sodium atom (symbol Na), which has 11 protons. According to the octet rule, what would be the simplest way for a sodium atom to achieve electron stability?
- Many elements have isotopes, which are rare variants of the element with additional neutrons in the nucleus. Deuterium is an isotope of hydrogen that has one neutron (normal hydrogen has no neutrons).
Does the neutron change the chemical reactivity of deuterium, compared with normal hydrogen? Explain why or why not.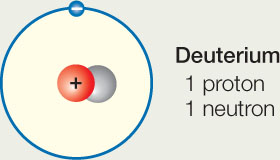
We have introduced the basic unit of matter that makes up all living organisms—the atom. We have discussed the tendency of atoms to attain stable configurations of electrons: a single shell of two electrons in the case of hydrogen, and an outer shell of eight electrons in the case of larger atoms. Next we will describe the different types of chemical bonds that can lead to stability, joining atoms together into molecular structures with different properties.